Abstract
This paper presents a new semi-quantitative metric, Green Star (GS), for evaluation of the global greenness of chemical reactions used in teaching laboratories. Its purpose is to help choose the more acceptable reactions for implementing Green Chemistry (GC) and to identify suitable modifications of protocols to improve the greenness of the chemistry practiced by students. GS considers globally, in principle, all the Twelve Principles of GC. The metric consists in the evaluation of the greenness of the reaction for each principle by pre-defined criteria, followed by graphical representation of the results in an Excel radar chart – the fuller the chart, the higher degree of greenness. To illustrate the construction and the scope of the metric, a case study is presented – the iron(II) oxalate dihydrate synthesis performed under several sets of conditions to pursue the implementation of greenness.
Introduction
The contribution of chemistry for pursuing sustainability is proactively performed by Green Chemistry (GC, in broad sense, including Green Chemical Engineering) and therefore chemistry should nowadays be taught under this new posture. The basic objectives of GC were defined in the Twelve Principles of GC, with a qualitative nature, defined by Anastas and Warner Citation1 and presented in .
Table 1. The Twelve Principles of GC.
To instill in students the new mentality for doing chemistry constituted by GC, the experimental study of chemistry must use new or modified experiments, especially synthesis experiments, with an intentional objective of increasing their greenness. For this purpose, we have asked some pre-service teachers to perform synthesis experiments as described in the literature (traditional synthesis) and then challenged them to modify the protocols to obtain greener synthetic procedures (“revised synthesis” for GC). The analysis, construction, and implementation of protocols to reach this purpose press the students to feel what GC is – and that it has to be pursued with a purposeful determination.
In this context, it is necessary to use metrics for quick evaluation of greenness to allow the comparison of traditional and revised procedures. Greenness is a complex feature that involves several different aspects of the compounds involved in the reactions and the reactions themselves – their safety, health, and environmental effects, etc. For the purpose of comparisons, mass metrics Citation2–6 (addressed to evaluate the accomplishment of the first two GC principles) and environmental metrics Citation7 Citation8 (addressed to measure the environmental benignness of the compounds and reactions, contemplated in the other principles) have to be used. However, the use of the simple metrics that have been introduced so far is not practical, as the variables to be considered in greenness are numerous, the number of metrics of both above types is large, their definition and calculation are complex, and the choice of the more suitable may be subjective and debatable. Therefore, metrics of larger scope addressing all the Twelve Principles of GC Citation1 in parallel are desirable.
The objective of this paper is to describe a new holistic metric developed for comparative evaluation of the greenness of reactions in undergraduate teaching laboratories, which we called Green Star (GS). GS was designed to address simultaneously all the principles of GC applicable in each situation.
To illustrate the construction of GS, the iron(II) oxalate dihydrate synthesis, performed in the laboratory under several sets of conditions to pursue increased greenness, is presented as a case study where GS is used for comparison of alternative procedures and identification of the most green. The new metric was evaluated against literature GC mass metrics.
The basic idea of Green Star (GS)
The Twelve Principles of GC are qualitative prescriptions and should be considered together in the evaluation of the greenness, because alterations of the conditions for executing the reactions may have different consequences with respect to different principles – the greenness may improve with reference to some of them but worsen with reference to others Citation9.
The basic idea of GS is to construct a star with a number of corners equal to the number of principles used for the evaluation of the synthesis reaction, all the 12 or only some if the remaining are not applicable, each corner with length proportional to the degree of accomplishment of the corresponding principle – a semi-quantitative view of the global greenness of the reaction can then be obtained by looking at the star and appreciating its area: the larger the area, the greener is the reaction.
Construction of Green Star (GS)
The construction of the metric consists in evaluating the greenness of the reaction for each principle (in a scale from 1 to 3, maximum value of greenness), by pre-defined criteria, followed by representing the results in an Excel radar chart – the fuller the chart, the higher degree of greenness.
The construction of the GS for a synthesis experiment begins by examining its protocol and making an inventory of all the substances involved: feedstock, products, by-products, auxiliary substances (catalytic reagents, solvents, separation agents, etc.) and, if possible, wastes. Next, to evaluate risks to human health and the environment and of potential chemical accident, the hazard symbols for each substance are collected, as well as information to identify whether the substances are renewable and break down into innocuous degradation products. According to this information, every substance is then classified in a scale from 1 to 3 by criteria chosen to be easy to use (see Tables ). For sake of safety, in case of lack of consistency of some of the information gathered about any of the items evaluated, the chosen value is that which most penalizes the item. The GS is then constructed giving the score 1, 2, or 3 to each of the Twelve Principles, following the criteria in .
Table 2. Risks to human health and environment of substances involved.
Table 3. Risks of potential chemical accident due to substances involved.
Table 4. Degradability and renewability characteristics of substances involved.
Table 5. Criteria and scores (S) to construct the GS.
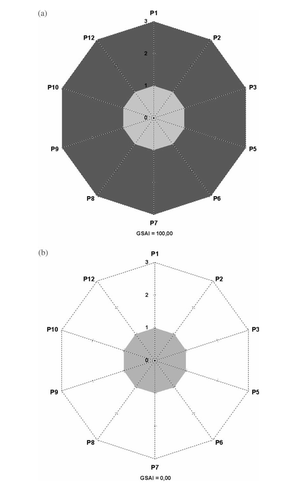
For maximum greenness, the score will be 3 for every principle and the area of the GS will be the fullest as presented in (a). On the other hand, when the greenness is minimum, all the scores will be 1 and the area of the GS will be minimum (zero, see below), as presented in (b). These two GS have only 10 corners, as the fourth and 11th principles were not considered, because teaching experiments do not usually include the preparation of new products (moreover, when dealing with chemical experiments that do not refer to synthesis, the GS will be reduced to six corners as the second, third, eighth, and ninth principles are not applicable).
In certain cases, when comparing GS for evaluation of relative greenness of alternative protocols, it may be difficult to evaluate which has a larger green area by visual inspection. To overcome this difficulty, a Green Star Area Index (GSAI) was included in the GS (see Appendix 1). The index is calculated as the ratio of the area of the GS to the area of the GS of maximum greenness, expressed as a percentage (100×area of the GS/area of GS of maximum greenness) and therefore varies between GSAI=100 (maximum greenness) and GSAI=0.
To illustrate the use of GS the iron(II) oxalate dihydrate synthesis is presented.
Synthesis of iron(II) oxalate dihydrate
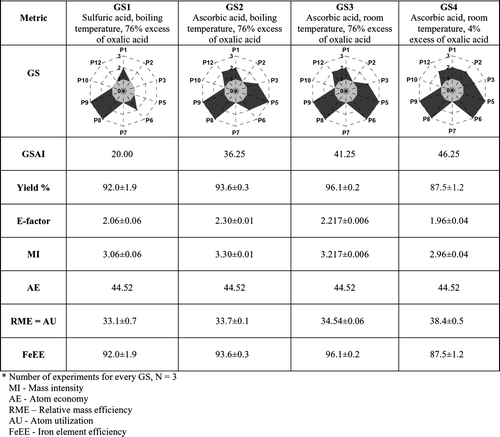
A number of experiments were initially performed following a published protocol Citation10 in which the product was prepared from iron(II) sulfate heptahydrate and oxalic acid dihydrate. This protocol proposed a large excess of oxalic acid, the use of sulfuric acid to acidify the iron(II) sulfate solution, and heating the mixture at temperature near the boiling point. To increase the greenness, the experiment was optimized by looking for: Citation1 more benign reagents – sulfuric acid was substituted by ascorbic acid to reduce iron(III), eventually formed, to iron(II); Citation2 energy efficiency – the experiments were performed at room temperature; and Citation3 stoichiometry – instead of large excess of oxalic acid, proportions close to stoichiometry were used to reduce waste (these also favor the metrics of incorporation of atoms into the product).
For each experiment, the GS was constructed from the data and several quantitative mass metrics were calculated Citation2–6: yield, E-factor, mass intensity (MI), atom economy (AE), atom utilization (AU), relaive mass efficiency (RME), and iron element efficiency (FeEE). The results were used to compare the response of GS and the other metrics when greenness increases.
Experimental procedure
First synthesis – traditional
A 5.0 g (18.0 mM) sample of iron(II) sulfate heptahydrate was dissolved in 25 mL of deionized water Citation10. The solution was acidified with 0.5 mL of 2 mol L–1 sulfuric acid. A solution of 4.0 g (31.7 mM) of oxalic acid dihydrate in 25 mL of deionized water was added and the mixture stirred, heated to the boiling temperature, and left to settle at room temperature. The yellow precipitate of iron(II) oxalate dihydrate was washed with hot water, suction filtered, washed again with hot water, and allowed to dry in the filter for about 10 minutes before being placed in a desiccator for about a week. An excess of about 76% of oxalic acid was prescribed in this protocol Citation10. Three experiments were performed and a 92% yield was obtained (92.0±1.9%, standard deviation).
The experiment was optimized in alternative procedures.
Second synthesis – first green improvement
To look for more benign reagents, 1 g (5.7 mM) sample of ascorbic acid was dissolved in 25 mL of deionized water and the iron(II) sulfate heptahydrate was dissolved in this solution (no sulfuric acid was used). After about 4 minutes, the oxalic acid was added. The mixture was heated to the boiling temperature and the initial protocol was then followed. Three experiments were performed with an excess 76% of oxalic acid and a 94% yield was obtained (93.6±0.3%).
Third synthesis – second green improvement
To increase the energy efficiency, experiments were performed at room temperature using this last protocol (it was verified that the solubility of iron(II) oxalate dihydrate increases with temperature). A 96% yield was obtained (three experiments, 96.1±0.2%).
Fourth synthesis – third green improvement
To look for proportions closer to stoichiometric, three experiments were performed at room temperature, with an excess of only 4% of oxalic acid. A 88% yield was obtained (three experiments, 87.5±1.2%).
To characterize the product, IR spectra (Mattson ATI Genesis Series FTIR, KBr pellets) were obtained for samples prepared by each protocol and for known samples (Aldrich #307726). It was verified that the IR spectra were similar.
Green Star (GS)
From data on properties, as referred previously (see discussion about Tables ), the risks for human health and for the environment of all the substances involved were collected in , from which the scores to construct the GS were obtained by the criteria in . The results are presented in .
Table 6. Risks for human health and for the environment for the synthesis of iron(II) oxalate dehydrate.
Table 7. Scores used to construct the GS for the synthesis of iron(II) oxalate dehydrate.
The GS of the experiments performed under different conditions are presented in . In this GSn denotes the GS for synthesis number (n=1–4, see above). The visual comparison of the four GS shows that:
-
When ascorbic acid was used instead of sulfuric acid (second synthesis), the scores for the third, the fifth, and the 12th principles increased, because ascorbic acid is less hazardous than sulfuric acid ( and ); as a result the green area of GS increased, GS2 is greener than GS1 ().
-
When the experiments were performed at room temperature (third synthesis), the energy efficiency was increased and the score for the sixth principle increased ( and ); as a result the green area of GS increased, GS3 is greener than GS2 ().
-
When conditions closer to stoichiometry were used (fourth synthesis), the excess of oxalic acid was reduced from 76 to 4%, therefore the incorporation of atoms from this reagent into the final product increased, although the incorporation of the atoms from the iron(II) sulfate into the final product decreased, as the yield decreased – indeed the increase of RME/AU () shows that the overall incorporation of atoms of reagents into the product increased; as a result, the score for the second principle increased and the green area of GS increased – GS4 is greener than GS3 ().
The values for GSAI included in confirm the visual evaluation of the GS. However, the green area (GSAI=46.25 for GS4) is far from the maximum.
Mass metrics (yield and GC mass metrics)
Using numerical data from the experiments, yields and the values of GC metrics were calculated as follows (see formula in Appendix 2). Water was not considered in the calculations Citation11 Citation12 because its inclusion makes comparisons between different protocols difficult. More precisely, as the mass of water is 10 times larger than the total mass of stoichiometric and other auxiliary reagents, the inclusion of water leads to values of E-factor and MI so high that mask the effects of the increase of the mass of the other auxiliary reagents along the experiments.
Waste minimization
-
E-factor, as the ratio of the total waste mass (total mass of reagents less mass of product) to the mass of product.
-
MI, as the ratio of the total mass of reagents (stoichiometric reagents, solvents, other auxiliary reagents, etc.) to the mass of product.
Incorporation of atoms of reagents into the product
The following metrics evaluate the incorporation of atoms of reagents into the product. This is the purpose of the second principle of GC.
-
AU, as the ratio of the mass of product to the mass of all the substances produced in the chemical reaction (product and by-products), expressed as a percentage.
-
AE, as the ratio of the mass of atoms of stoichiometric reagents that are incorporated in the final product (molecular weight of the product) to the mass of total atoms of stoichiometric reagents (sum of the molecular weights of stoichiometric reagents), as a percentage (it was assumed that there were no losses in the process and that all stoichiometric reagents have been converted to product and by-products).
RME, as the ratio of the mass of product to the mass of stoichiometric reagents, as a percentage.
FeEE, as the ratio of the mass of iron in the product to the mass of total iron present in reagents, as a percentage.
The values of RME and AU were the same, as AU was calculated considering the mass of the waste and not the mass of by-products. The metrics RME and element EE (in the present case FeEE) are used in industrial processes and have been useful to evaluate the incorporation of atoms from the reagents into the product, because their calculation is easy Citation12.
The results are included in . The values show that when ascorbic acid was used (second synthesis), a fuller GS (GS2) was obtained than for the traditional synthesis (GS1) and the GSAI increased (20.00→36.25). When the GC mass metrics are considered, the E-factor increased slightly (2.06→2.30) as well as MI (3.06→3.30), due to the increase of the mass of auxiliary substances, therefore the loss of atoms in wastes increased. At the same time, the values of AU, RME, and FeEE also increased slightly, therefore the incorporation of atoms from the reagents into the product also increased. These two conclusions are apparently contradictory: while the E-factor and MI suggest that the experiments are less green, the AU, RME, and FeEE indicate that the experiment is greener. This false contradiction may be explained as follows: (1) the use of the innocuous auxiliary substances had a negative influence on the E-factor and MI because the total mass of auxiliary substances was increased; and (2) the yield increased and this had a positive influence on AU, RME, and FeEE, which are calculated considering only stoichiometric reagents. In summary, the increase of greenness was detected by GS, but was not perceived by the values of GC mass metrics, as their results are contradictory.
The values in also show that for experiments at room temperature (third synthesis), the GS (GS3) is fuller than for the second synthesis (GS2), the GSAI increased (36.25→41.25); with reference to the mass metrics, the E-factor and MI decreased slightly and AU, RME, and FeEE increased slightly. These variations in the GC mass metrics are due to an increase in the yield, as these metrics do not respond to the energy efficiency.
Finally, when experiments were performed near stoichiometry (fourth synthesis), the GS (GS4) was found to be fuller than for the previous case (GS3), the GSAI increased (41.25→46.25) and both the E-factor (2.22→1.96) and MI (3.22→2.96) decreased. At the same time, the values of AU/RME (34.5→38.4) increased, although the yield decreased about 9%. In this case, the increase of greenness (productive use of atoms) was confirmed by the values of the mass metrics. When this final protocol is compared with the initial, it was found that the GSAI increased to more than twice its initial value (20.00→46.25) and the E-factor decreased (2.06→1.96), MI decreased (3.06→2.96), AU and RME increased (33.1→38.4).
The value of AE is the same for all experiments, as it is calculated for theoretical conditions, considering that there were no losses in the process and that all stoichiometric reagents were converted to product and by-products.
The increase in greenness along the green synthesis optimization procedure is better observed in the GS animation (online edition).
Discussion
Comparisons of the results of GS (evaluation of benignness) with GC mass metrics (evaluation of the efficient use of atoms in a chemical reaction) seem to indicate that the two types of metrics, when used together, allow a fuller initial evaluation of the green quality of a synthesis process. Indeed, GS and GC mass metrics provide different but complementary indications about the greenness.
With reference to the waste formed, GS considers only its deleterious nature, while the E-factor and MI evaluate the mass of waste, which is an important factor for loss of greenness. However, when GC mass metrics are considered alone, the results can be misleading, for example, if a small mass of very toxic waste is produced, a low value of the E-factor is obtained, although the high toxicity is very deleterious to greenness.
The same happens when solvents and other auxiliary substances are at play, as GS considers their nature but GC mass metrics do not distinguish between toxic or innocuous solvents, evaluating only the masses involved. This is particularly relevant when the solvent is water. The water quantity used, although often discarded in calculations, may be evaluated through GC mass metrics, but as it is an innocuous solvent, it has no effects in GS.
The substitution of reagents for more benign ones may have a negative impact on GC mass metrics if that implies a larger mass, a decrease of the yield and/or a waste increase. The yield of a chemical reaction has a high influence on the GC mass metrics, but none directly in GS.
The improvement of the greenness of a process may imply the increase of its costs, but this may not be the case if the amount of waste and the cost of waste treatment and disposal, together with potential risks of chemical accidents, are considered. The optimization of the GS may imply less favorable GC mass metrics, but the economic calculation of alternatives should consider environmental costs.
In summary, the advantage of GS is that it allows one to consider aspects which are not dealt with by mass metrics: (i) energy efficiency (sixth principle); (ii) the use of renewable feedstocks (seventh principle); (iii) reduction of derivatives (eighth principle); (iv) the use of catalysts and their toxicity (ninth principle); (v) the degradation of the substances involved (tenth principle); and (vi) risks to human health and to the environment caused by the nature of the substances (first, third, fifth, ninth, and 12th principles).
Conclusion
These results seem to indicate that GS is useful to evaluate the greenness of synthetic procedures, although more experiments involving other synthesis are required to test other factors which may affect it (further studies of other cases are now under progress). This conclusion is supported by several GS characteristics:
-
GS may be used to evaluate of the greenness of a chemical reaction without performing the experiment, from a protocol, if enough detail is provided in it.
-
GS allows the comparison of the greenness of different alternative experimental procedures by mere visual analysis, although a number between 1 and 100 can be used as result of the metric (GSAI).
-
GS allows easy identification by visual analysis of the aspects that should be optimized to improve greenness.
-
GS is easy to construct, although sometimes it may be difficult to obtain at the start all the information needed, specially about the degradability of the substances involved.
-
GS responds holistically to a large number of features that must be considered when the greenness of a chemical reaction is under discussion, as it deals with all the relevant Twelve Principles of GC in a global and systematic way.
The incorporation of GC in the teaching environment is adequate to help develop a new look on chemistry by the students, hopefully more optimistic than the present, without compromising the integrity of chemistry knowledge. It is important that students change their posture to look for optimization of the greenness of chemical reactions, and when designing and performing several experiments under different conditions for this purpose, the GS seems to be a useful metric.
Acknowledgements
The authors wish to thank the pre-service teacher M. Salomé Fernandes for her collaboration in the experiments for preparation of iron(II) oxalate dihydrate.
References
- Anastas , P.T. and Warner , J.C. 1998 . Green Chemistry – Theory and Practice , Oxford, UP : Oxford .
- Sheldon , R.A. Chem. Ind . 1992 , 903 906 .
- Sheldon , R.A. Chem. Tech . 1994 , 24 , 39 47 .
- Trost , B.M. Science 1991 , 254 , 1471 1477 .
- Curzons , A.D. ; Constable , D.J.C. ; Mortimer , D.N. ; Cunningham , V.L. Green Chem . 2001 , 3 , 1 6 .
- Constable , D.J.C. ; Curzons , A.D. ; Santos , L.M.F. ; Green , G.R. ; Hannah , R.E. ; Hayler , J.D. ; Kitterindham , J. ; McGuire , M.A. ; Richardson , J.E. ; Smith , P. ; Webb , R.L. ; Yu , M. Green Chem . 2001 , 3 , 7 9
- Frosch , R.A. , Industrial Environmental Performance Metrics – Challenges and Opportunities ; Washington, DC : National Academy Press , 1999 .
- Allen , D.T. and Shonnard , D.R. 2002 . Green Engineering – Environmentally Conscious Design of Chemical Processes , Upper Saddle River, NJ : Prentice-Hall .
- Blackmond , D.G. ; Armstrong , A. ; Coombe , V. ; Wells , A. Angew. Chem. Int. Ed . 2007 , 46 , 3798 3800 .
- Pass , G. and Sutcliffe , H. 1974 . Practical Inorganic Chemistry , Chapman and Hall : London .
- Sheldon , R.A. Green Chem . 2007 , 9 , 1273 1283 .
- Constable , D.J.C. ; Curzons , A.D. ; Cunningham , V.L. Green Chem . 2002 , 4 , 521 527
Appendix 1. Calculation of the Green Star (GS) area and Green Star Area Index (GSAI)
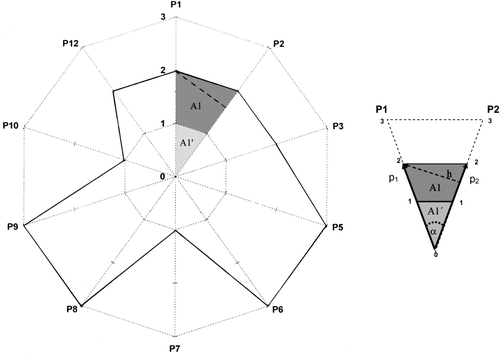
The calculation of GSAI is exemplified for the case of GS4 in the text (see ). The calculation is based on the expression for the area of the triangle (see insert in ). For the branch of the star between the P1 and P2 axes (scores p 1 and p 2, respectively) the area of the triangle defined by the scores p 1 and p 2 (triangle 022, area A1′+ A1, base p2, height h,), as h=p 1 sin α, is:
Similarly, for the central triangle 011 (light colored), for which p 1=p 2=1, the area is
Therefore, the contribution to the area of the GS (dark colored trapezoid) is:
The total area of GS is calculated by addition of these areas for the 10 branches of the star:
where the sum index refers to the axes (not the principles). For maximum greenness all the scores would be p i =3 and the area of the full star would be:
therefore,
Appendix 2. Calculation of Green Chemistry (GC) mass metrics for a chemical reaction
A+B→P+D
In the above equation, A and B represent stoichiometric reagents, P the product, and D the by-products. In the formula below, m w represents the mass of total waste, m P the mass of the product, m A and m B the masses of the stoichiometric reagents, m D the mass of by-products, m aux the mass of auxiliary reagents, AWFe the atomic weight of iron, MWA, MWB, and MWP the molecular weights of stoichiometric reagents and the product and n FeP, n FeA, n FeB the number of iron atoms in the molecular formula of the product and of the stoichiometric reagents, respectively. The GC mass metrics were calculated using the formulae presented below.E-factor