Abstract
A facile and eco-friendly methodology for the synthesis of N-alkyl/aralkyl derivatives of indole & indole-3-carboxyaldehydes mediated by polyethylene glycol as an efficient and green solvent is described.
Keywords:
Introduction
Indole and its myriad of derivatives continue to capture the attention of synthetic organic chemists due to their profound biological activity Citation1. In view of their importance as potential pharmacological compounds we intended to prepare N-substituted derivatives of simple indoles and indole-3-aldehydes. Herein we report the results of those studies in this communication.
Previous methods used to synthesize N-substituted indoles Citation2–5 and indole-3-aldehydes Citation6 were associated with long reaction times, tedious workup procedures and low yields. In addition, the reaction media were found to be solvents such as benzene, acetonitrile, and dimethyl formamide; all of which are toxic, carcinogenic or non-eco-friendly in nature. Hence, it was our endeavor to replace the reaction medium and develop an eco-friendly methodology for syntheses of N-substituted derivatives in reasonably good yields.
Results and Discussion
Polyethylene glycol (PEG-600) promoted reactions Citation7–10 have attracted attention of chemists due to their ease of workup, the ability to act as phase transfer catalysts and their inexpensive and eco-friendly nature. Hence, we explored the possibilities of exploiting the versatile features of this (PEG-600) green solvent. In this connection, we are reporting a synthesis of N-substituted indole-3-aldehydes in the presence of PEG-600 as a facile and versatile reaction medium at 120–130°C without additional usage of any base.
Earlier it was reported Citation11 from our lab that the reaction of indole-3-carboxyaldehyde with alkylating agents in the presence of K2CO3 as a base and TEBAC as phase transfer catalyst in acetonitrile/dimethylformamide reaction medium for 4–6 hrs at room temperature resulted in the formation of the corresponding N-alkyl derivatives of indole-3-aldehyde in good to moderate yields. When we replace the reaction medium with PEG-600 without additional usage of base the time taken to complete the reaction decreased to 2–3 hrs, also resulting in higher yields. In order to compare the rate of the reaction in PEG-600, we carried out the reaction in different solvents ().
Table 1. Rate of reaction in different solvents.
PEG-600 is a very effective solvent which dissolves the substrate (i.e. indole-3-carboxyaldehyde) and the reagent (i.e. alkylating agent) bringing them together thereby providing an effective means for chemical reaction to occur. Further, PEG-600 is able to extract the hydrogen from the − NH of indole-3-carboxyldehyde and is able to retain it by chelation through several lone pairs of electrons in its oxygen containing chain. This role of PEG-600 is very similar to that of proton sponge (i.e. 1, 8-dimethylaminonaphthalene) which is a very strong base due to its ability to extract hydrogen from an acidic substrate and then retain it in its claws by chelation through lone pair of electrons on the two nitrogen atoms of proton sponge.
Stimulated by the ease of reaction, various indoles and indole-3-aldehyde derivatives were made to react with different alkylating agents in the presence of PEG-600, to yield N-alkyl derivatives in excellent yields ( and ). In conclusion, we have developed an inexpensive, fast, and eco-friendly methodology for the synthesis of N-substituted derivatives of indole and indole-3-aldehydes using PEG-600 as the reaction medium.
Table 2. Analytical and spectral data of N-alkyl derivatives of indole-3-carboxyaldehyde.
Table 3. Characterization data of compounds prepared.
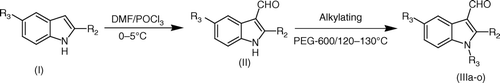
Indole-3-aldehyde was synthesized from indole using Vilsmeir-Haack formylation Citation12. The obtained product was heated with the suitable alkylkating agent (DMS, DES, PhCH2Cl, etc.) in PEG-600 as a reaction medium at 120–130°C without additional usage of any base followed by a simple workup to yield the N-alkyl derivatives of indole-3-aldehyde. The reaction was extended to other indole-3-aldehyde derivatives to build the library of N-substituted indole-3-aldehyde derivatives. All the above compounds were characterized by 1H NMR, IR, and CI mass spectra.
Experimental
Melting points were measured in open capillary tubes and are uncorrected. TLC was done on glass plates coated with Silica Gel – G and spotting was done using iodine or UV lamp. IR spectra were recorded using Perkin Elmer model-446 FTIR in KBr. 1H NMR spectra were recorded on a Gemini-2000 and AV-400 operating at 200 and 400 MHz, respectively.
Preparation of II from I
To DMF (1.6 ml, 22 mmol) cooled in ice-water, was added POCl3 (0.05 ml, 5.5 mmol) during 30 minutes. The solution was stirred at the same temperature for an additional 15 minutes until an orange syrupy liquid was formed. To this, I (5 mmol) in DMF (10 ml) was added over 30 minutes. The solution was stirred at 0–5°C for an additional 20 minutes and brought to RT. The mixture was stirred at RT for 45 minutes. After this period, the solution was poured into crushed ice and neutralized with three portions of NaOH (4 g in 20 ml), the two portions being added drop wise maintaining the pH at acidic and third one was added in one lot and solution was heated on a water bath for period of 1–2 minutes when a solid began to separate. The separated solid was filtered, washed with water and dried to obtain crude II. The latter was recrystalized from ethanol to obtain pure II.
Preparation of III from II: (General procedure)
About 5 mM of the appropriate indole-3-aldehyde derivative was taken in 50 ml round bottom flask containing 25 ml of PEG-600. To this was added the appropriate alkylating agent (DMS, DES, PhCH2Cl, etc.) (7.5 mmol) dropwise at RT. The reaction mass was refluxed for 2 hr at 120–130°C. At the end of this period, the reaction mass was poured into ice cold water. The separated solid was filtered, washed with excess water and dried to obtain the crude. The latter were recrystalized from methanol to obtain pure III.
Acknowledgements
The authors are highly indebted to University Grants Commission, Govt. of India, New Delhi, for providing financial support. They are also thankful to the authorities of Jawaharlal Nehru Technological University, Hyderabad, for providing laboratory facilities.
References
- Sundberg , R.J. The Chemistry of Indoles ; Academic Press : New York, London , 1970 .
- Plieninger , H. Chem. Ber . 1954 , 87 , 127 128 .
- Cornforth , R.H. ; Robinson , R. J. Chem. Soc . 1940 , 680 683 .
- Pederson , E.B. ; Zahran , M.A. ; Afify , H.M. ; Nielsen , C. J. Chem. Res . ( S ) 2001 , 9 , 101 104 .
- Chauhan , S. Indian J. Chem . 1994 , 33B , 32 37 .
- Smith , G.F. J. Chem. Soc . 1954 , 3842 3846 .
- Dicekerson , T.J. ; Janda , K.D. Chem. Rev . 2002 , 102 , 3325 3344 .
- Kamal , A. Reddy , D.R. Rajender . Tetrahedron Lett . 2005 , 46 , 7951 7953 .
- Babasaheb Bandgar , P. ; Balaji Korea , L. ; Sachin Patil , A. ; Sunita Bandgar , B. ; Hemant Chavan , V. ; Baliram Hote , S. Aust. J. Chem . 2008 , 61 9 , 700 703 .
- Mahalle , S. ; Dinesh , L. ; Ramrao , M. Green Chem. Lett. Rev . 2008 , 2 , 103 106 .
- Dubey , P.K. ; Balaji , B. ; Venkatanarayana , M. Indian J. Heterocycl. Chem . 2006 , 15 , 205 208 .
- James , P.N. ; Snyder , H.R. Org. Synth . 1959 , 39 , 30 33 .
- Blume , R.C. ; Lindwall , H.G. J. Org. Chem . 1945 , 10 , 255 258 .
- Noland , W.E. ; Reich , C. J. Org. Chem . 1967 , 32 , 828 832 .
- Ottoni , O. ; Cruz , R. ; Alves , R. Tetrahedron 1998 , 54 , 13915 13928 .
- Da Settimo , A. ; Saettone , M.F. Tetrahedron 1965 , 21 , 1923 1929 .