Abstract
The 2,4,6-trichloro-1,3,5-triazine catalyzed synthesis of β-amino alcohols by aminolysis of epoxide under solvent-free condition is described. Mild reaction conditions, short reaction times, inexpensive and readily available catalysts, and excellent yields of the products with high regioselectivity are attractive features of this methodology.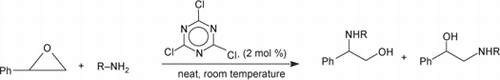
Introduction
β-Amino alcohols are versatile intermediates in the synthesis of biologically active natural products, unnatural amino acids, β-blockers as well as insecticidal agent, and chiral auxillaries Citation1–6. Classically, β-amino alcohols were synthesized by aminolysis of epoxides through heating with an excess of amine. This classical method has a number of limitations, such as the requirement of an excess of inorganic base, longer reaction times, low nucleophilicity in the case of deactivated aromatic amines, and in some cases low boiling points when elevated temperature is necessary Citation7. Even though some of these drawbacks have been overcome with the use of a variety of catalysts, such as Bi(OTf)3 Citation8, InBr3 Citation9, Cu(BF4)4·xH2O Citation10, H3PW12O40 Citation11, CeCl3·7H2O Citation12, SmI2 Citation13, [Bmin]BF4 Citation14, Alumina Citation15, DIPAT Citation16, COCl2 Citation17, ZnCl2 Citation18, βCD/H2O Citation19, there are still many limitations, such as the formation of bis-alkylated products, longer reaction times Citation8 Citation9 Citation11–18, stoichiometric amounts of catalysts Citation9 Citation12 Citation14–16 Citation19, and harsh reaction conditions Citation15 Citation18. However, some of these reported methods are not applicable for aliphatic amines Citation8 Citation9 Citation11–14 Citation17. The formation of complex metal salts having strong Lewis acid properties with aliphatic amines causes catalyst poisoning and makes the metal salts unsuitable as catalysts for the opening of epoxide rings by aliphatic amines. Therefore, highly efficient reagents which can catalyze this transformation with low catalyst loading and under mild conditions are still desirable.
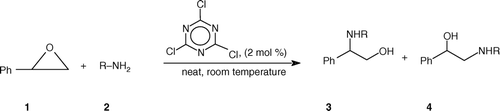
Over the last few years, there has been a considerable growth in interest in the use of 2,4,6-trichloro-1,3,5-triazine (cyanuric chloride) or its derivatives Citation20–34 in organic synthesis. Recent results from our laboratory had shown that simple and readily available cyanuric chloride can assist some important organic reactions Citation33 Citation34. In continuation to our ongoing program, we would like to report a mild, practical, and efficient method for the opening of epoxides with substituted amines using catalytic amounts of cyanuric chloride under solvent-free conditions ().
Results and discussion
Initially a systematic study was carried out for the catalytic evaluation of cyanuric chloride for the reaction of styrene oxide with aniline under various conditions (). The reaction between styrene oxide and aniline in the absence of a catalyst produced only a trace amount of product after 20 h (, entry 1) and inferior results were obtained with CH2Cl2, THF, CH3CN, and CHCl3 (, entries 2–5). We then optimized the quantity of catalyst at room temperature under solvent-free conditions for this reaction (, entries 6–11). It was observed that the use of just 2 mol% of cyanuric chloride is sufficient for the completion of the reaction in 15 min with 98% yield of the corresponding product (entry 9). It is important to note that larger amounts of catalyst did not improve the results (, entries 10–11). With less than 2 mol% of the catalyst, the reaction was incomplete and resulted in low yield of the product (, entries 6–8).
Table 1. Reaction of styrene oxide with aniline under various conditions.
Styrene oxide underwent cleavage by a range of aromatic primary amines (, entries 1–3) and aliphatic amines (, entries 4–6) in a regioselective manner with preferential attack at the benzylic and less-hindered terminal carbon of styrene oxide, respectively.
Table 2. Cyanuric chloride catalyzed synthesis of β-amino alcohols.
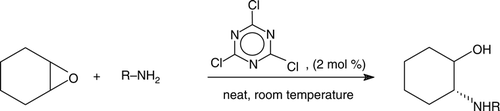
In the case of cyclic oxides, the reaction worked well independently of ring size and gave exclusively the corresponding trans-amino alcohols (, entries 7–9). The relative stereochemistry was determined based on the coupling constants of the peaks at Δ3.11 (CHNHPh; ddd, J=4.0, 9.1, and11.1 Hz) and Δ3.34 ppm (CHOH; ddd, J=4.0, 9.7, and 9.7 Hz) in their 1H NMR spectra ().
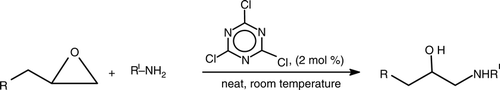
Finally, the aliphatic epoxides were treated with amines (). In each case, preferential nucleophilic attack at the terminal carbon of the epoxide ring was observed with quantitative yield.
This methodology is also compatible with functionalities, such as OH, OTBDMS, OTHP, CO2Me, and Br (, entries 16–20). These reactions are highly selective for the formation of β-amino alcohols as the only products in excellent yields keeping the intact other functionalities.
The comparison of the present method with respect to the amount of catalyst and amine, reaction time and temperature, requirement of solvent and the product yield with those of literature reports reveals that this newly developed method is superior to the reported ones. Another important feature of this methodology is cyanuric chloride effectively catalyzes the reaction of opening of epoxides with aliphatic or aromatic amines, which are compatible with those of literature, reported methods Citation8 Citation9 Citation11–14 Citation17.
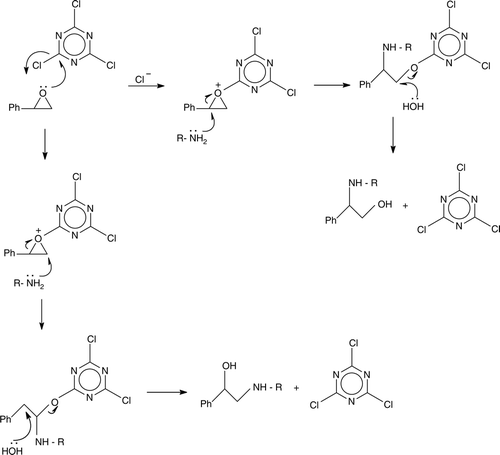
We proposed the possible role played by cyanuric chloride in the ring opening reaction as follows ().
In conclusion, the present method is a novel, mild, and efficient for the opening of epoxides with amines. The experimental simplicity, mild reaction conditions, reduced reaction times, inexpensive catalyst, and high yields of the products with excellent regioselectivities, which makes it potentially useful for the industrial applications.
Experimental
The 1H NMR spectra were recorded in CDCl3 at 300 MHz using TMS as internal standard. IR spectra were recorded using KBr pellets for solids and neat for liquid samples. Column chromatography was performed using silica gel (100–200 mesh). Chemical shifts are given in ppm with respect to internal TMS and J values are quoted in Hz.
General reaction procedure
A mixture of epoxides (1 mmol), anilines (1 mmol), and cyanuric chloride (2 mol%) was stirred at room temperature under solvent-free condition. After completion of the reaction (TLC), the reaction mixture was washed with 0.5 N HCl (15 ml) and extracted with diethyl ether (3×10 ml). The combined organic layer was dried over anhydrous Na2SO4. The solvent was evaporated and the crude product purified by column chromatography on silica gel (Merck 60–120 mesh, petroleum ether: ethyl acetates = 9:1) to afford pure product.
Spectroscopic data of new compound
Bisoprolol (, entry 13): oil, IR (CHCl3): 2969, 2866, 1612, 1585, 1468, 1367, 1174, 1093, and 822 cm−1; 1HNMR (CDCl3): 7.28 (d, J=8.4 Hz, 2H), 6.9 (d, J=8.4 Hz, 2H), 4.50 (s, 2H, OCH2), 4.0 (m, 5H), 3.58 (m, 4H), 2.68–2.90 (m, 2H), 2.29 (brs, 1H, OH), 1.18 (d, J=5.7 Hz, 6H), and 1.10 (d, J=6.3 Hz, 6 H); Mass (FAB): m/z=312 (M++1); analysis calculated for C17H29O4N: C, 65.56; H, 9.38; and N, 4.49; Found: C, 65.52; H, 9.34; and N, 4.44.
References
- Ager , D.J. ; Prakash , I. ; Schad , D. Chem. Rev . 1996 , 96 , 835 876 .
- Goodman , L.S. ; Gilman , A. The Pharmacological Basic of Therapeutics , 6th ed Macmillan : New York , 1980 ; 1 6 .
- Rogers , G.A. ; Parson , S.M. ; Anderson , D.C. ; Nilsson , L.M. ; Bhar , B.A. ; Kornereich , W.D. ; Kaufman , R. ; Jacobs , R.S. ; Kirtman , B. J. Med. Chem . 1989 , 32 , 1217 1230 .
- Auvin-Gutte , C. ; Rebuffat , S. ; Prigent , V. ; Bodo , B. J. Am. Chem. Soc . 1992 , 114 , 2170 2174 .
- Wu , S. ; Takeya , R. ; Eto , M. ; Tomizawa , C. J. Pestic. Sci . 1987 , 12 , 221 227 .
- Corey , E.J. ; Bakshi , R.K. ; Shibata , S. ; Chen , C.P. ; Sing , V.K. J. Am. Chem. Soc . 1987 , 109 , 7925 7926 .
- Deyrup , J.A. ; Moyer , C.L. J. Org. Chem . 1969 , 34 , 175 179 .
- Ollevier , T. ; Compin , G. Tetrahedron Lett . 2004 , 45 , 49 52 .
- Rodriguez , J.R. ; Navarro , A. Tetrahedron Lett . 2004 , 45 , 7495 7498 .
- Kamal , A. ; Ramu , R. ; Azar , M.A. ; Ramesh Khanna , G.B. Tetrahedron Lett . 2005 , 46 , 2675 2677
- Babu , K.S. ; Raju , B.C. ; Praveen Kumar , S. ; Mallur , S.G. ; Reddy , S.V. ; Rao , J.M. Synth. Commun . 2005 , 35 , 879 885 .
- Reddy , L.R. ; Reddy , M.A. ; Bhanumati , N. ; Rao , K.R. Synthesis 2001 , 6 , 831 832 .
- Carree , F. ; Gil , R. ; Collin , J. Tetrahedron Lett . 2004 , 45 , 7749 7751 .
- Yadav , J.S. ; Reddy , B.V.S. ; Basak , A.K. ; Narsaiah , A.V. Tetrahedron Lett . 2003 , 44 , 1047 1050 .
- Harrak , Y. ; Pujol , M.D. Tetrahedron Lett . 2002 , 43 , 819 822 .
- Rampalli , S. ; Chaudhari , S.S. ; Akamanchi , K.G. Synthesis 2000 , 1 , 78 80 .
- Iqbal , J. ; Pandey , A. Tetrahedron Lett . 1990 , 31 , 575 576 .
- Pachon , L.D. ; Gamez , P. ; Vanbrussel , J.J.M. ; Reedijk , J. Tetrahedron Lett . 2003 , 44 , 6025 6027 .
- Surendra , K. ; Krishnaveni , N.S. ; Rama Rao , K. Synlett 2005 , 3 , 506 510 .
- Venkatraman , K. ; Wagle , D.R. Tetrahedron Lett . 1979 , 32 , 3037 3040 .
- Kaminski , Z.J. Synthesis 1987 , 17 , 917 920 .
- Kunishima , M. ; Morita , J. ; Kawachi , C. ; Iwasaki , F. ; Terao , K. ; Tani , S. Synlett 1999 , 8 , 1255 1256 .
- Kaminska , J.E. ; Kaminski , Z.J. ; Gora , J. Synthesis 1999 , 4 , 593 596 .
- Rayle , H.L. ; Fellmeth , L. Org. Procs. Res. Devp . 1999 , 3 , 172 176 .
- Falorni , M. ; Giacomelli , G. ; Porcheddu , A. ; Taddei , M. J. Org. Chem . 1999 , 64 , 8962 8964 .
- De , L. ; Luca , S. ; Giacomelli , G. ; Porcheddu , A. Org. Lett . 2001 , 3 , 1519 1521 .
- Samaritani , R. ; Menicagli , R. Tetrahedron 2002 , 58 , 1381 1386 .
- Falorni , M. ; Porcheddu , A. ; Taddei , M. Tetrahedron Lett . 1999 , 40 , 4395 4396 .
- Forbes , D.C. ; Barrett , E.J. ; Lewis , D.J. ; Smith , M.C. Tetrahedron Lett . 2000 , 41 , 9943 9947 .
- De Luca , L. ; Giacomelli , G. ; Porcheddu , A. J. Org. Chem . 2002 , 67 , 5152 5155 .
- Blothy , G. Tetrahedron Lett . 2003 , 44 , 1499 1501 .
- Karimi , B. ; Hazarkhani , H. Synthesis 2003 , 16 , 2547 2551 .
- Bandgar , B.P. ; Joshi , N.S. ; Kamble , V.T. ; Sawant , S.S. Aust. J. Chem . 2008 , 61 , 231 234
- Bandgar , B.P. ; Joshi , N.S. ; Kamble , V.T. Tetrahedron Lett . 2006 , 47 , 4775 4777 .