Abstract
An environmentally benign protocol for the synthesis of chalcones by the Claisen–Schmidt condensation of aldehydes with ketones using eco-friendly non-toxic bismuth(III)chloride catalyst under solvent-free condition is reported. In this protocol, the reaction time is very short, yields are high, and there are no other pollutants formed.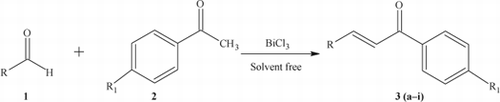
Introduction
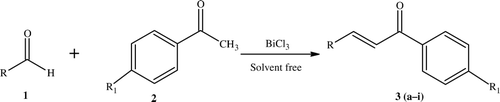
Chalcones are normally produced by the Claisen–Schmidt condensation of aldehydes with ketones. The chalcone motif is widely distributed in natural products Citation1 and they are crucial intermediates for the synthesis of a variety of pharmaceuticals. These molecules have a broad spectrum of biological activities, such as anti-bacterial Citation2, antioxidant Citation3, anti-inflammatory Citation4, antimalarial Citation5, antileshmanial Citation6, anticancer Citation7, and antitumor Citation8. In the synthesis of chalcones, various caustic, alkali, and clay catalysts have been used, including aqueous NaOH Citation2 Citation9, KOH Citation10–12, Ba(OH)2 Citation13 hydrotalcites, zeolites Citation14, LiHDMS Citation15, and calcined NaNO3/natural phosphates Citation16 Citation17, AlCl3 Citation18, dry HCl Citation19, Zn(bpy)(OAc)2 Citation20, TiCl4 Citation21, Cp2ZrH2/NiCl2 Citation22, RuCl3 Citation23, ZnCl2 Citation24 , and Al2O3–AlPO4 Citation25. Recently, BF3·Et2O Citation26 Citation27, SOCl2/EtOH Citation28, ultrasound accelerated activated carbons (Na and Cs-Norit) Citation29, molecular I2 Citation30, and Bronsted acidic ionic liquid catalysts Citation31. Because of the non-toxic nature and mild Lewis acid activity of bismuth(III)chloride, we and others have been using this as an efficient green catalyst for the production of vital organic compounds Citation32–40. In this communication, described herein is the synthesis of chalcones employing bismuth(III)chloride as a mild, green, and efficient catalyst ().
Results and discussion
We initiated our investigation with model experiments using 5 mol% BiCl3 in the condensation of benzaldehyde (5 mmol) and acetophenone (5 mmol) under solvent-free conditions at 140°C (it was found that lower and higher temperatures were not suitable). It was found that any reduction in the amount of catalyst used resulted in a decrease in yield. In order to optimize the yields, experiments were carried out with varying levels of catalyst. It was established that 10 mol% of BiCl3 is the optimum concentration. In a typical procedure, benzaldehyde, acetophenone, and BiCl3 (1:1:0.1) were mixed thoroughly and stirred at the appropriate temperature under solvent-free conditions for 20 minutes to obtain the product 3 in high yields. To check the efficacy and applicability of this catalyst, the reaction was generalized using various aldehydes and acetophenones (). As is clear from , the reaction has wide applicability and the reaction times are only 10–20 minutes. The mechanism of the BiCl3 catalyst is that it activates the methyl group of the ketones used by attaching itself to the carbonyl oxygen, as is typical in similar reactions. In comparison to earlier reported catalysts, the BiCl3 catalyst is inexpensive, non-toxic, and uses mild reaction conditions. In this present protocol, a variety of chromonyl chalcones (3d, 3f, and 3i) have been synthesized and the obtained yields are very high in the comparison to previously reported processes (). In the case of compounds 3d, 3f, and 3i, the reaction temperature was maintained at 110°C since at higher temperatures the chromone moiety breaks down.
Table 1. BiCl3 catalyzed condensation of aldehydes and ketones.
Experimental
Melting points(MP) were determined in open capillaries and are uncorrected. Reagent-grade chemicals were purchased from commercial source and used without further purification. IR spectra were recorded in KBr discs on a Perkin–Elmer 240C analyzer. 1H NMR spectra were recorded on Varian Gemini 300 (300-MHz) spectrometer using trimethyl silane as internal standard. The progress of the reaction was monitored by thin layer chromatography (TLC) using silica gel G (Merck).
General procedure for preparation of chalcones
A mixture of benzaldehyde (10 mmol, 1.06 g), acetophenone (10 mmol, 1.20 g), and BiCl3 (0.1 mmol) was heated at 140°C for 20 minutes under solvent-free condition. The reaction was monitored via TLC using toluene:ethylacetate (9:1). After completion of the reaction, the reaction mixture is cooled to room temperature and product is isolated to obtain crude product, which is re-crystallized from absolute alcohol to afford the pure chalcone.
Physical and spectral data
In the case of 3-(3-aryl-3-oxo1-propenyl)-4H-1-benzopyran (3d), the yield is 90% and the absorption observed in IR region is 1682 cm−1 for O = C–C and 1638 cm−1 for pyronyl C = O, respectively. In the 1H NMR spectra, a pair of doublets at δ 7.11 and δ 7.87 was found for the α and β protons of chalcone system, the hydrogen attached to C2 position of chromone appeared as a singlet at δ = 8.26, and other aromatic protons appeared as a complex multiplet pattern at δ = 7.22–8.12, respectively.
3-(4-Ethoxyphenyl)-1-phenylpropenone
MP 52°C (52–53)30; IR (KBr): 3021, 2927, 1656, 1596, 1216, and 760 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.01 (d, J=7.2 Hz, 2H), 7.79 (d, J=15.9 Hz, 1H), 7.57–7.38 (m, 6H), 6.89 (d, J=7.9 Hz, 2H), 4.01 (q, J=7.5, 6.8 Hz, 2H), and 1.40 (t, J=6.7 Hz, 3H); 13C NMR (75 MHz): δ= 190.48, 161.20, 144.74, 138.52, 132.66, 130.30, 128.59, 128.43, 127.37, 119.50, 114.93, 63.52, and 14.54; ESI (m/z) 252 [M + H]+ (, product 3b).
3-(2-Methoxyphenyl)-1-phenylpropenone
MP 52–53°C (53–54)30; IR (KBr): 3061, 2956, 2837, 1659, 1597, 1340, 1248, 1209, 1018, and 753 cm−1; 1H NMR (CDCl3, 300 MHz): δ= 8.17 (d, J=15.4 Hz, 1H), 8.04 (d, J=7.7 Hz, 2H), 7.67–7.45 (m, 5H), 7.34 (t, J=7.7 Hz, 1H), 6.99–6.94 (t, J=7.7 Hz, 1H), 6.89 (d, J=9.0 Hz, 1H), and 3.84 (s, 3H); 13C NMR (75 MHz): δ = 191.01, 158.96, 140.51, 138.55, 132.79, 132.01, 129.26, 128.61, 128.53, 123.90, 122.72, 120.89, 111.34, and 55.62; ESI (m/z) 239 [M + H] + (, product 3c).
3-(4-Ethoxyphenyl)-1-p-tolylpropenone
MP 94–95°C (95–96)30; IR (KBr): 3030, 2977, 1648, 1563, and 1217 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.94 (d, J=7.6 Hz, 2H), 7.79 (d, J=15.2 Hz, 1H), 7.57 (d, J=8.7 Hz, 2H), 7.41 (d, J=15.2 Hz, 1H), 7.27 (d, J=7.6 Hz, 2H), 6.90 (d, J=8.7 Hz, 2H), 4.02 (q, J=7.5, 6.8 Hz, 2H), 1.40 (t, J=7.6 Hz, 3H), and 2.1 (s, 3H); 13C NMR (75 MHz): δ = 189.87, 161.11, 144.33, 143.33, 135.90, 130.18, 129.28, 128.61, 127.47, 119.46, 114.84, 63.70, 21.71, and 14.85; ESI (m/z) 267 [M + H]+ (, product 3e).
1-(4-Chlorophenyl)-3-(4-ethoxyphenyl)-propenone
MP 125°C (125–126)30; IR (KBr): 3019, 1657, 1601, 1216, 1028, and 759 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.95 (d, J=9.0 Hz, 2H), 7.77 (d, J=15.7 Hz, 1H), 7.56 (d, J=7.9 Hz, 2H), 7.44 (d, J=7.8 Hz, 2H), 7.35 (d, J=15.8 Hz, 1H), 7.31 (d, J=8.9 Hz, 2H), 4.05 (q, J=7.5, 6.8 Hz, 2H), and 1.42 (t, J=6.7 Hz, 3H); 13C NMR (50 MHz): δ = 189.79, 162.06, 146.03, 139.32, 137.26, 130.8, 130.29, 129.3, 127.65, 119.31, 115.35, 64.15, and 15.17; ESI (m/z) 287 [M + H]+ (, product 3g).
1-(4-Chlorophenyl)-3-(2-methoxyphenyl)-propenone
MP 75–76°C (75–76)30; IR (KBr): 3017, 2935, 1655, 1599, 1501, 1218, 1168, 1026, and 758 cm–1; 1H NMR(CDCl3, 300 MHz): δ= 8.13 (d, J=14.8 Hz, 1H), 7.94 (d, J=9.2 Hz, 2H), 7.61 (d, J=8.3 Hz, 1H), 7.54 (s, 1H), 7.42 (d, J=7.3 Hz, 2H), 7.36 (t, J=7.4 Hz, 1H), 6.99–6.89 (m, 2H), and 3.88 (s, 3H); 13C NMR (50 MHz): δ = 190.06, 159.44, 141.23, 139.29, 137.18, 132.47, 130.4, 129.69, 129.25, 124.06, 122.56, 121.24, 111.68, and 55.93; ESI (m/z) 273 [M + H]+ (, product 3h).
Conclusion
In summary, the present method employing BiCl3 is mild, efficient, and environment benign green protocol for the synthesis of chalcones. The products are obtained in high yields and the reaction time is short. The present protocol employs a catalyst that is inexpensive and a well-known non-toxic inorganic salt. Furthermore, the process is carried out with operational simplicity and simple work-up procedures. These features place this protocol at an advantage to the existing processes.
Acknowledgements
The authors are thankful to the Council of Scientific and Industrial Research, New Delhi, India, for financial assistance and to the Indian National Science Academy, New Delhi, India, for additional financial support for this research project.
References
- Oliver , K. and Albrecht , F.K. 2001 . Phytother. Res , 15 : 148 – 152 .
- Nielsen , S.F. , Christensen , S.B. , Cruciani , G. , Kharazmi , A. and Liljefors , T. 1998 . J. Med. Chem , 41 : 4819 – 4832 .
- Mukherjee , S. , Kumar , N. , Parasad , A.K. , Raj , H.G. , Bracke , M.E. , Olsen , C.E. , Jain , S.C. and Parmar , V.S. 2001 . Bioorg. Med. Chem , 9 : 337 – 345 .
- Hsieh , H.K. , Tsao , L.T. , Wang , J.P. and Lin , C.N. 2000 . J. Pharm. Pharmacol , 52 : 163 – 171 .
- Ram , V.J. , Saxena , A.S. , Srivastava , S. and Chandra , S. 2000 . Bioorg. Med. Chem. Lett , 10 : 2159 – 2161 .
- Zhai , L. , Chen , M. , Blam , J. , Theander , T.G. , Chiristensen , S.B. and Kharazmi , A. 1999 . J. Antimicrob. Chemother , 43 : 793 – 803 .
- Anto , R.J. , Sukumuran , K. , Kuttan , G. , Rao , M.N.A. , Subbaraju , V. and Kuttan , R. 1995 . Cancer Lett , 97 : 33 – 37 .
- Kumar , S.K. , Hager , E. , Catherine , P. , Gurulingappa , H. , Davidson , N.E. and Khan , S.R. 2003 . J. Med. Chem , 46 : 2813 – 2815 .
- Dimmock , J.R. , Kandepu , N.M. , Hetherington , M. , Quail , J.W. , Pugazhenthi , U. , Sudom , A.M. , Chamankhah , M. , Rose , P. , Pass , E. , Allen , T.M. , Halleran , S. , Szydlowski , J. , Mutus , B. , Tannous , M. , Manavathu , E.K. , Myers , T.G. , Clercq , E.D. and Balzarini , J. 1998 . J. Med. Chem , 41 : 1014 – 1026 .
- Bu , X. ; Zhao , L. ; Li , Y. Synthesis . 1997 , 1246 1248 .
- Shankar , M.S.S. , Reddy , R.B. , Chandra Mouli , G.V.P. and Reddy , Y.D. 1989 . J. Indian Chem. Soc , 66 : 30 – 31 .
- Bu , X. and Li , Y.G. 1996 . J. Nat. Prod , 59 : 968 – 969 .
- Alcantara , A.R. , Marinas , J.M. and Sinisterra , J.V. 1987 . Tetrahedron Lett , 28 : 1515 – 1518 .
- Climent , M.J. , Corma , A. , Iborra , S. and Primo , J. 1995 . J. Catal , 15 : 60 – 66 .
- Daskiewicz , J.B. , Comte , G. , Barron , D. , Pietro , A.D. and Thomasson , F. 1999 . Tetrahedron Lett , 40 : 7095 – 7098 .
- Sebti , S. , Solhy , A. , Tahir , R. , Boulaajaj , S. , Mayoral , J.A. , Fraile , J.M. , Kossir , A. and Oumimoun , H. 2001 . Tetrahedron Lett , 42 : 7953 – 7955 .
- Sebti , S. , Solhy , A. , Smahi , A. , Kossir , A. and Oumimoun , H. 2002 . Catal. Commun , 3 : 335 – 339 .
- Calloway , N.O. and Green , L.D. 1937 . J. Am. Chem. Soc , 59 : 809 – 811 .
- Sz'ell , T. and Sohalr , I. 1969 . Can. J. Chem , 47 : 1254 – 1258 .
- Irie , K. and Watanabe , K. 1980 . Bull. Chem. Soc. Jpn , 53 : 1366 – 1371 .
- Mazza , L. ; Guaram , A. Synthesis . 1980 41 44
- Nakano , T. , Irifune , S. , Umano , S. , Inada , A. , Ishii , Y. and Ogawa , M. 1987 . J. Org. Chem , 52 : 2239 – 2244 .
- Iranpoor , N. and Kazemi , F. 1998 . Tetrahedron , 54 : 9475 – 9480 .
- Reddy , G.V. , Maitraie , D. , Narsaiah , B. , Rambabu , Y. and Rao , P.S. 2001 . Synth Commun , 39 : 2881 – 2884 .
- Cabello , J.A. , Campelo , J.M. , Garcia , A. , Luna , D. and Marinas , J.M. 1984 . J. Org. Chem , 49 : 5195 – 5197 .
- Narender , T. and Papi Reddy , K. 2007 . Tetrahedron Lett , 48 : 3177 – 3180 .
- Huang , D.F. , Wang , J.X. and Hu , Y.L. 2003 . Chin. Chem. Lett , 14 : 333 – 334 .
- Petrov , O. , Ianova , Y. and Gerova , M. 2008 . Catal Commun , 9 : 315 – 316 .
- Calvino , V. , Picallo , M. , Lopez-Peinado , A.J. , Martin-Aranda , R.M. and Duran-Valle , C. 2006 . J. Appl. Sur. Sci , 252 : 6071 – 6074 .
- Sashidhara , K.V. , Rosaiah , J.N. and Kumar , A. 2009 . Synth. Commun , 39 : 2288 – 2296 .
- Shen , J. , Wang , H. , Lia , H. , Sun , Y. and Liu , Z. 2008 . J. Mol. Catal. A: Chem , 280 : 24 – 28 .
- Baruah , B. ; Baruah , A. ; Prajapati , D. ; Sandhu , J.S. Tetrahedron Lett . 1997 , 38 , 1449 1450 .
- Borah , H.N. , Prajapati , D. , Sandhu , J.S. and Ghosh , A.C. 1994 . Tetrahedron Lett , 35 : 3167 – 3170 .
- Boruah A. Baruah B. Prajapati D. Sandhu J.S. Synlett 1997 1251 1252
- Thakur , A.J. , Boruah , A. , Prajapati , D. and Sandhu , J.S. 2000 . Synth. Commun , 30 : 2105 – 2111 .
- Wada , M. , Ohki , H. and Akiba , K.Y. 1990 . Bull. Chem. Soc. Jpn , 63 : 1738 – 1747 .
- Suzuki , H. ; Ikegami , T. ; Matano , Y. Synthesis . 1997 , 249 267 .
- Vidal , S. Synlett . 2001 , 1194 1195 .
- De , S.K. ; Gibbs , R.A. Synthesis . 2005 , 1231 1233 .
- Ramalinga , K. ; Vijayalakshmi , P. ; Kaimal , T.N.B. Synlett . 2001 , 863 865 .