Abstract
ZnO-beta zeolite, an inexpensive and mild catalyst, has been used for the synthesis of polyhydroquinolines in excellent yields from the one-pot four-component condensation of aldehydes, dimedone, ethyl acetoacetate, and ammonium acetate in ethanol at room temperature. The remarkable advantages offered by this method are a green catalyst, mild reaction conditions, simple work-up procedures, much faster reactions, and excellent yield of products. Furthermore, the catalyst could be reused several times keeping its initial activity in recycled reactions.
Introduction
Zeolite beta is a high silica zeolite, containing an intersecting three-dimensional structure of 12-membered ring channels. Due to this relatively voluminous channel structure, it is possible to carry out numerous acid-catalyzed reactions effectively Citation1 . Furthermore, zeolite beta also has adjustable acidity in the protonic form and this is another factor affecting the efficiency of reaction. Zeolite beta has been found to be a suitable transesterification catalyst to synthesize a variety of products Citation2 . It is clear that the transesterification proceeds on the Bronsted acidic sites of the zeolite beta and the Bronsted acid sites of the zeolite beta also can be subtly adjusted by modification with metal cations, bringing about modified catalysts with suitable acidity to fit different transesterifications Citation3–7 .
For economic and environmental reasons, the use of heterogeneous solid acid catalysts to achieve effective catalyst handling, easy isolation of products, and reusability is continuously increasing. Among the various solid acid catalysts, zeolites have received an increasing amount of attention because of their suitable acidity, thermal stability, easy work-up, recyclability, and eco-friendly nature. Zeolites have been used as catalysts in the petroleum refining and chemical industries Citation8 Citation9 . Their properties and thus, their performance as catalysts, can be adjusted by modification by ion exchange with metal ions, acid treatment, and hydrothermal treatment Citation10 Citation11 . In particular, zinc-loaded zeolites are suitable for various organic transformations, such as the Heck reaction Citation12, propane aromatization Citation13 , dehydrogenation of small paraffins Citation14, aromatization of in situ generated ethylene Citation15, and the hydration of acetylene Citation16 Citation17.
Heterocyclic compounds generally exhibit raised biological activity and they also make possible the development of novel materials with unique properties. An interesting and promising class of heterocycle is the 1,4-dihydropyridines (1,4-DHPs). This type of heterocycle has significant pharmacological and biological activities, such as vasodilator, bronchodilator, anti-atherosclerotic, anti-tumor, geroprotective, hepatoprotective, and antidiabetic agents Citation21 Citation22 . 1,4-DHPs are analogues of NADH coenzymes and an important class of blockers of calcium (Ca2 +) channel Citation23 Citation24 . These examples make clearly explicit the remarkable potential of novel currents DHPs derivatives as a source of valuable drug candidates.
Due to their great importance, many synthetic strategies have been employed for the synthesis of polyhydroquinolines, such as iodotrimethylsilane (TMSI) Citation25, Yb(OTf)3 Citation26, promotion of microwave Citation27, ceric ammonium nitrate (CAN) Citation28, potassium dodeca tungsto cobaltate trihydrate Citation29, molecular iodine Citation30, HClO4–SiO2 Citation31, l-Proline Citation32, Ni-nanoparticle Citation33, montmorillonite K10 Citation34, and HY zeolite Citation35 . Furthermore, many of these methods require either a long reaction time or harsh reaction conditions or the use of expensive catalyst. Therefore, the development of simple, efficient, and general methodology for this one-pot four-component reaction is still desirable.
Moreover, to the best of our knowledge no report has been made about the use of zinc-modified beta zeolite as a catalyst for the Hantzsch condensation. By considering the importance of polyhydroquinolines and as a part of our ongoing investigation in developing a versatile and efficient method for synthesis of heterocyclic compounds Citation36–39 . Herein, we report a very simple, fast, and general method for the synthesis of polyhydroquinolines via Hantzsch condensation of aldehydes, dimedone, ethyl acetoacetate, and ammonium acetate in the presence of ZnO-beta zeolite ().
Results and discussion
X-ray diffraction (XRD) analysis
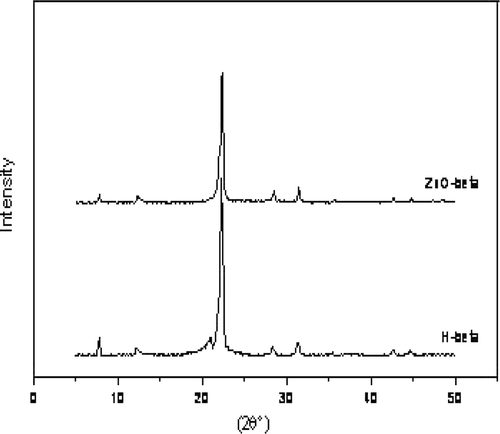
depicts the X-ray diffraction (XRD) pattern of H-beta and ZnO-beta zeolite. The powder XRD pattern is the typical type for beta zeolite by comparing it with the reference beta zeolite Citation40 . It was observed that, there is no visible difference between synthetic beta zeolite and zinc-modified beta zeolite. It is clear that samples contain typical diffraction peaks of H-beta zeolite (2θ = 7.8° and 22.3°).
Fourier transform infrared (FT-IR) analysis
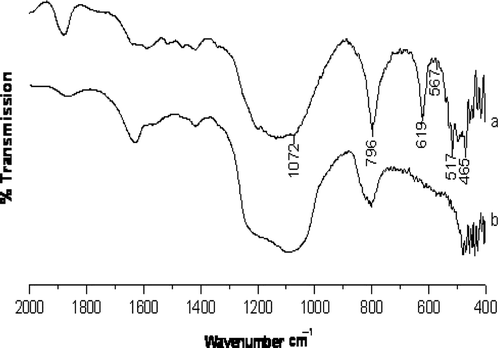
The Fourier transform infrared (FT-IR) spectra of H-beta zeolite and ZnO-beta zeolite are shown in . Synthesized material showing IR bands in the range 550–600 cm−1 usually indicates the presence of zeolite-like material. Peaks around 575 and 525 cm−1 (567 and 517 cm−1 in present work) correspond to H-beta zeolite Citation41 . The characteristic vibrations of H-beta zeolite around 465 and 427 cm−1 are also observed. In zinc-modified beta zeolite only the disappearance of peak at 619 cm−1 is observed.
Scanning electron microscope–energy dispersive X-ray spectroscopy (SEM–EDS) analysis
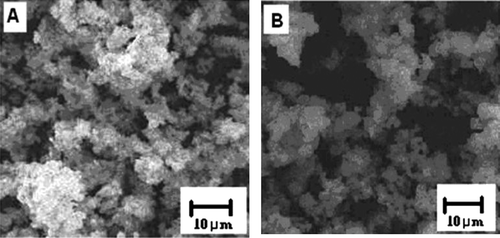
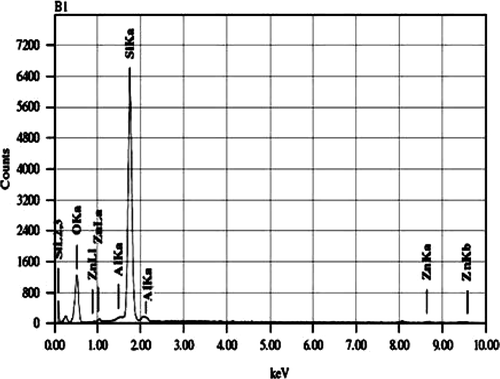
The morphology of the zeolite beta and ZnO-beta zeolite are summarized in . The scanning electron microscope (SEM) image shows that the interconnected porous structure by agglomerating of tiny particles of ZnO on H-beta zeolite crystals with an average particle size less than 10 µm (B). From energy dispersive X-ray spectroscopy (EDS) analysis zinc content in modified beta zeolite is 2.75 mass% ().
NH3–temperature-programmed desorption (NH3–TPD) and Brunnauer–Emmett–Teller (BET) analysis
Temperature-programmed desorption (TPD) measurements were carried out by Citation1 pre-treating of samples from room temperature to 200°C and gas flow of nitrogen; Citation2 adsorption of ammonia at room temperature; (3) desorption of adsorbed ammonia with an heating rate 10°C min−1 starting from the adsorption temperature to 700°C. Total acidity and calculated BET surface area of ZnO-beta zeolite is summarized in .
Table 1. The acid strength and surface area of catalyst.
In order to get the best experimental conditions, we initially studied the effect of various solvents and catalytic efficiency of ZnO-beta zeolite for the synthesis of ethyl 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-5-oxo-4-phenylquinoline-3-carboxylate (, entry 4a) using the model reaction of benzaldehyde (1 mmol), dimedone (1 mmol), ethyl acetoacetate (1 mmol), and ammonium acetate (1.5 mmol). The reaction in acetone, toluene, and chloroform which give low yields after 5, 8, and 6 h, respectively, are given in (entries 1–3) while acetonitrate and methanol gave moderate yield of products (, entries 4, 5). A quantitative yield of desired product was obtained in the presence of 0.1 gm of ZnO-beta zeolite within 30 min. indicating that the 0.1 gm of ZnO-beta zeolite in EtOH system is a very active catalytic system for this reaction. It is noteworthy that in the absence of catalyst, reaction gave only 18% yield at room temperature after 12 h.
Table 2. Optimization of reaction conditions for the synthesis of Ethyl 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-5-oxo-4-phenylquinoline-3-carboxylate.a
With optimized reaction condition in hand, we have synthesized various polyhydroquinoline derivatives (, entries 4 (a–m)) via the condensation of substituted aldehydes with dimedone, ethyl acetoacetate, and ammonium acetate in shorter reaction times (30–60 min) with excellent yields (86–95%). This methodology avoids the use of hazardous solvents and requires only catalytic amount of the ZnO-beta zeolite to promote the reaction.
Table 3. Synthesis of polyhydroquinoline derivatives catalyzed by ZnO-beta zeolite.a
We have successfully used recovered ZnO-beta zeolite for the same model reaction and the results of recycling experiments are shown in . These results clearly indicate that the recovered ZnO-beta zeolite can be recycled successfully without significant loss of activity.
Table 4. Reusability of ZnO-beta zeolite catalyst for the synthesis of compound 4a (, entry 4a).a
In we compared our result with results obtained by a reported procedure for the synthesis of compound 4a. The data presented in this table show the promising feature of this method in terms of reaction rate and the yield of product compared with that reported in the literature.
Table 5. Comparative data of ZnO-beta with other catalyst for the synthesis of 4a (, entry 4a).a
Experimental
All the chemicals were purchased from Merck, S.D. Fine India and used without further purification. Thin layer chromatography (TLC) was performed on Merck-precoated silica gel 60-F254 plates. The X-ray powder diffraction patterns of catalyst were recorded by using Bruker 8D advance X-ray diffractometer using Cu–Kα radiation of wavelength = 1.54056 Å. FT-IR spectra of catalyst were recorded on JASCO FT-IR-4100, Japan. SEM image with EDS was obtained on JEOL, JSM-6330 LA operated at 20.0 kV and 1.0000 nA. BET surface area has been measured by means of N2 adsorption at 77 K preformed on a Quantachrome CHEMBET 3000 instrument. TPD measurements were carried out on a Quantachrome CHEMBET 3000 TPR/TPD instrument. All products are known compounds and their physical data, FT-IR, 1H NMR, and mass spectra were essentially identical with those of authentic samples.
Preparation of catalyst
In a typical synthesis, tetraethyl orthosilicate (TEOS) was added to a mixture of tetraethyl ammonium hydroxide (TEAOH), sodium hydroxide (NaOH), and an aqueous solution of aluminum sulfate (Al2(SO4)3) and stirred at room temperature for 24 h. This mixture was then hydrothermally treated at 120°C for 96 h in an autoclavable bottle. After this mixture was cooled to room temperature, the solid material obtained was filtered and washed with deionised water, dried at 80°C for 6 h and calcined at 550°C for 12 h. H-form of beta zeolite was prepared through ion exchange of the above sample with 1 M ammonium acetate solution at 80°C for 10 h. The ion exchange procedure was repeated twice and the resulting product was calcined at 550°C for 8 h. Beta zeolite was modified by mixing H-form of respective zeolite with an aqueous solution of zinc acetate. The mixture was digested at 80°C for 8 h, dried and calcined at 550°C for 8 h.
Typical reaction procedure for synthesis of polyhydroquinolines
A mixture of aldehyde (1 mmol), dimedone (1 mmol), ethyl acetoacetate (1 mmol), ammonium acetate (1.5 mmol), and ZnO-beta zeolite (0.1 gm) were added in ethanol (5 mL). The reaction mixture was stirred at room temperature until the reaction was completed (monitored by TLC). After completion of the reaction, the reaction mixture was dissolved by heating, then the reaction mass filtered. The undissolved material, i.e. catalyst was washed by n-hexane (2×5 mL), dried at 80°C further used for next reaction Citation42 Citation43 . The filtrate was concentrated under reduced pressure and the obtained solid was recrystallized from ethanol to obtain pure product.
Spectroscopic data of some compounds
Ethyl 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-5-oxo-4-phenylquinoline-3-carboxylate (4a): 1H NMR (80 MHz, CDCl3): Δ 0.94 (s, 3H), 1.09 (s, 3H), 1.14 (t, J=7.3 Hz, 3H), 2.13–2.34 (m, 4H), 2.37 (s, 3H), 4.05 (q, J=7.3 Hz, 2H), 5.02 (s, 1H), 5.74 (s, 1H), 7.03–7.34 (m, 5H); 13C NMR (75 MHz, DMSO-d6) Δ 14.2, 19.1, 21.3, 27.6, 36.5, 37.3, 59.8, 106.0, 113.7, 126.3, 127.8, 128.0, 143.3, 147.1, 149.2, 167.3, 194.8; IR (KBr in cm−1): 3233, 3210, 3080, 1696, 1602, 1059, 692; m/z = 340 (M + H)+.
Ethyl 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-4-(3-nitrophenyl)-5-oxoquinoline-3-carboxylate (4i): 1H NMR (80 MHz, CDCl3): Δ 0.96 (s, 3H), 1.04 (s, 3H), 1.22 (t, J=7.3 Hz, 3H), 2.10–2.34 (m, 4H), 2.38 (s, 3H), 4.01 (q, J=7.3 Hz, 2H), 4.96 (s, 1H), 6.32 (s, 1H), 6.74–7.38 (m, 4H); 13C NMR (75 MHz, DMSO-d6) Δ 14.18, 19.32, 21.1, 27.3, 33.1, 33.90, 59.5, 105.4, 112.3, 121.2, 122.8, 128.6, 134.8, 144.6, 148.3, 149.5, 151.0, 166.9, 196.0; IR (KBr in cm−1): 3303, 2954, 1683, 1610, 1167, 759; m/z = 385 (M + H)+.
Ethyl 1,4,5,6,7,8-hexahydro-4-(4-methoxyphenyl)-2,7,7-trimethyl-5-oxoquinoline-3-carboxylate (4b): 1H NMR (80 MHz, CDCl3): Δ 0.96 (s, 3H), 1.06 (s, 3H), 1.22 (t, J=7.2 Hz, 3H), 2.10–2.26 (m, 3H), 2.34–2.40 (m, 4H), 3.77(s, 3H), 4.02 (q, J=7.2 Hz, 2H), 5.08 (s, 1H), 5.85 (s, 1H), 6.71–7.24 (m, 4H); 13C NMR (75 MHz, DMSO-d6) Δ 14.3, 17.9, 26.3, 28.8, 32.4, 35.0, 50.1, 50.4, 55.1, 59.2, 102.7, 109.5, 113.4, 128.3, 128.5, 140.0, 144.9, 149.1, 156.8, 168.2, 193.8; IR (KBr in cm−1): 3281, 3199, 3080, 1708, 1607, 1224, 837; m/z = 370 (M + H)+.
Conclusions
In summary, this paper describes a simple, convenient, and eco-friendly protocol for the one-pot multicomponent synthesis of polyhydroquinoline derivatives in ethanol at room temperature. This method offers very attractive features, such as short reaction times (30–60 min), excellent yields, and easy work-up procedure. Moreover, the ZnO-beta zeolite was successfully reused for four cycles without significant loss of activity. It is thus a rapid, convenient work-up procedure, mild reaction conditions, inexpensive, and eco-friendly nature of the catalyst that makes this method a valid contribution to the existing methodologies.
Acknowledgements
The authors are thankful to the Head, Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad for providing the laboratory facility.
References
- Jansen , J.C. and Creyghton , E.J. 1997 . Catal. Today , 38 : 205 – 212 .
- Sasidharan , M. and Kumar , R. 2004 . J. Mol. Catal. A Chem. , 210 : 93 – 98 .
- Bortnovsky , O. , Sobalik , Z. and Wichterlova , B. 2001 . Micropor. Mesopor. Mater. , 46 : 265 – 275 .
- Ramos , M.J. , Casas , A. , Rodríguez , L. , Romero , R. and Perez , A. 2008 . Appl. Cata. A. , 346 : 79 – 85 .
- Penzien , J. , Abraham , A. and Bokhoven , J.A.V. 2004 . J. Phys. Chem. , 108 : 4116 – 4126 .
- Valverde , J. , Lucas , A.D. and Lez , M.G. 2001 . J. Chem. Eng. Data. , 46 : 1404 – 1409 .
- Samanta , S. , Giri , S. and Sastry , P.U. 2003 . Ind. Eng. Chem. Res. , 42 : 3012 – 3018 .
- Breck , D.W. Zeolite Molecular Sieves ; Wiley : New York , 1974 .
- Dyer , A. An Introduction to Zeolite Molecular Sieves ; Wiley : Chichester , 1988 .
- Dorado , F. ; Romero , R. ; Canizares , P. Appl. Catal. A 2002 , 236 , 235 243 .
- Shu , Q. , Yang , B. , Yuan , H. , Qing , S. and Zhu , G. 2007 . Catal. Comm. , 8 : 2159 – 2165 .
- Djakovitch , L. and Koehler , K. 2000 . J. Am. Chem. Soc. , 123 : 5990 – 5999 .
- Biscardi , J.A. , Meitzner , G.D. and Iglesia , E. 1998 . J. Catal. , 179 : 192 – 202 .
- Ono , Y. 1992 . Catal. Rev. Sci. Eng. , 34 : 179
- Hagen , A. ; Roessner , F. Studies in Surface Science and Catalysis ; Krager , H.G. ; Weitkamp , J. Elsevier : Amsterdam, The Netherlands , 1999 98 182 183
- Onyestak , G.Y. ; Kallo , D. Studies in Surface Science and Catalysis ; Delmon , B. ; Froment , G.F. Amsterdam, , The Netherlands : Elsevier , 1987 34 605 612 .
- Onyestak , G.Y. ; Papp , J. ; Kallo , D. Studies in Surface Science and Catalysis ; Karge , H.G. ; Weitkamp , J. Amsterdam, , The Netherlands : Elsevier , 1989 46 241 249
- Zhu , J. ; Bienayme , H. Multicomponent Reactions ; Wiley-VCH : Weinheim, , Germany , 2005 .
- Domling , A. ; Ugi , I. Angew. Chem. Int. Ed . 2000 , 39 , 3168 3210 .
- Fayol , A. ; Zhu , J. Org. Lett . 2005 , 7 , 239 242 .
- Bossert , F. ; Meyer , H. ; Wehinger , E. Angew. Chem. Int. Ed. Engl . 1981 , 20 , 762 769 .
- Nakayama , H. ; Kasoaka , Y. Heterocycles 1996 , 42 , 901 909 .
- Godfraid , T. ; Miller , R. ; Wibo , M. Pharmocol. Rev . 1986 , 38 , 321 416 .
- Mannhold , R. ; Jablonka , B. ; Voigdt , W. ; Schoenafinger , K. ; Schravan , K. Eur. J. Med. Chem . 1992 , 27 , 229 235 .
- Sabitha , G. ; Reddy , G.S.K.K. ; Reddy , C.S. ; Yadav , J.S. Tetrahedron Lett . 2003 , 44 , 4129 4131 .
- Wang L.M. Sheng J. Zhang L. Han J.W. Fan Z.Y. Tian H. Qian C.T Tetrahedron 2005 61 1539 1543
- Tu , S.J. ; Zhou , J.F. ; Deng , X. ; Cai , P.J. ; Wang , H. ; Feng , J.C. Chin. J. Org. Chem . 2001 , 21 , 313 316 .
- Reddy , C.S. ; Raghu , M. Chin. Chem. Lett . 2008 , 19 , 775 779 .
- Nagarapu , L. ; Apuri , S. ; Gaddam , S. ; Bantu , R. ; Mahankhali , V.C. ; Kantevari , S. Lett. Org. Chem . 2008 , 5 , 60 64 .
- Akbari , J.D. ; Tala , S.D. ; Dhaduk , M.F. ; Joshi H.S. Arkivoc 2008 , xii , 126 135 .
- Maheswara , M. ; Siddaiah , V. ; Damu , G.L.V. ; Rao , C.V. Arkivoc 2006 , ii , 201 206 .
- Karade , N.N. ; Budhewar , V.H. ; Shinde , S.V. ; Jadhav , W. Lett. Org. Chem . 2007 , 4 , 16 19 .
- Sapkal , S.B. ; Shelke , K.F. ; Shingate , B.B. ; Shingare , M.S. Tetrahedron Lett . 2009 , 50 , 1754 1756 .
- Song , G. ; Wang , B. Synth. Commn . 2005 , 35 , 2875 2880 .
- Das , B. ; Ravikanth , B. ; Ramu , R. ; Rao , V.B. Chem. Pharm. Bull . 2006 , 54 , 1044 1045 .
- Gadekar , L.S. ; Katkar , S.S. ; Vidhate , K.N. ; Arbad , B.R. ; Lande , M.K. Bull. Catal. Soc. India 2008 , 7 , 79 86 .
- Gadekar , L.S. , Arbad , B.R. and Lande , M.K. 2008 . Org. Chem. Ind. J. , 4 : 458 – 461 .
- Shinde , S.V. ; Jadhav W.N. ; Lande , M.K. ; L.S. Gadekar .; Arbad , B.R. ; Kondre J.M. ; Karade , N.N. Catal. Lett . 2008 , 125 , 57 61 .
- Gadekar , L.S. ; Mane , S.R. ; Katkar , S.S. ; Arbad , B.R. ; Lande , M.K. Cent. Eur. J. Chem . 2009 , 7 , 550 554 .
- Shen , B. ; Wang , P. ; Yi , Z. ; Zhang , W. ; Tong , X. ; Liu , Y. ; Guo , Q. ; Gao , J. ; Xu , C. Energy Fuels 2009 , 23 , 60 64 .
- Perez-Pariente , J. ; Martens , J.A. ; Jacobs , P.A. Appl. Catal . 1987 , 31 , 35 64 .
- Das , B. , Ravikanth , B. , Ramu , R. and Rao , B.V. 2006 . Chem. Pharm. Bull. , 54 : 1044 – 1045 .
- Gadekar , L.S. , Katkar , S.S. , Mane , S.R. , Arbad , B.R. and Lande , M.K. 2009 . Bull. Korean Chem. Soc. , 30 : 2532 – 2534 .