Abstract
Inhibitive and adsorption properties of albomycin as a green inhibitor for the corrosion of zinc in H2SO4 solutions were studied using weight loss and hydrogen evolution methods. The results obtained, indicate that albomycin is a good adsorption inhibitor for the corrosion of zinc in H2SO4 solution. The inhibition potentials of albomycin for the corrosion of zinc in H2SO4 solutions are attributed to the adsorption of the inhibitor on the surface of zinc and its inhibition efficiency increases with increase in the concentration of the inhibitor but decreases with increasing temperature. The range obtained for the values of activation energy and free energy of adsorption were within the limit expected for the mechanism of physical adsorption. Also, the adsorption of albomycin on zinc surface was spontaneous, exothermic, and supported the Langmuir adsorption model. Optimized structure of 2-[[2-[[5-[acetyl(hydroxy)amino]-2-[[5-[acetyl(hydroxy)amino]-2-[[5-[acetyl(hydroxy)amino]-2-aminopentanoyl]amino] pentanoyl] amino] pentanoyl]amino]-3-hydroxypropanoyl]amino]-3-[(2R,3S,4R,5R)-3,4-dihydroxy-5-(3-methyl-2,4-dioxopyrimidin-1-yl)thiolan-2-yl]-3-hydroxypropanoic acid; iron(3 + ) (albomycin)
Optimized structure of 2-[[2-[[5-[acetyl(hydroxy)amino]-2-[[5-[acetyl(hydroxy)amino]-2-[[5-[acetyl(hydroxy)amino]-2-aminopentanoyl]amino] pentanoyl] amino] pentanoyl]amino]-3-hydroxypropanoyl]amino]-3-[(2R,3S,4R,5R)-3,4-dihydroxy-5-(3-methyl-2,4-dioxopyrimidin-1-yl)thiolan-2-yl]-3-hydroxypropanoic acid; iron(3 + ) (albomycin)
Keywords:
Introduction
Corrosion inhibitors are needed in the oil, fertilizer, and metallurgical industries because when metals (such as zinc, mild steel, aluminium, etc.) come in contact with aggressive solutions (such as acids, bases, and salts), the rate at which they corrode is reduced by the adsorption of these inhibitor on their surfaces Citation1–6.
Numerous studies have been carried out on the inhibition of the corrosion of metals in acidic and alkaline media Citation7–10. Most of these studies are centered on the use of organic compounds as inhibitors. These organic compounds have suitable functional groups, conjugated systems, or centers for pi-bond, which facilitate the adsorption of the inhibitor on the metal surface. It has also been established that the presence of aromatic groups and hetero atoms also enhance the adsorption potentials of these groups of inhibitors Citation11 Citation12. Based on their structures, some of the inhibitors that have been used for the corrosion of zinc are quinoline, aniline, ephedrine, narcotine, brucine, stryctuine, piperazine, caffine, barbitone, sparfloxacin, some surfactant, benzotriazole, norfloxacin, thiourea, quinolones, azithromycin, and pyridine derivatives Citation13–17. Attempts have also been made to use some naturally occurring substances as inhibitors for the corrosion of metals including zinc Citation18–20. Inhibitors from this source are called green corrosion inhibitors because they are environmentally friendly and do not contain heavy metals or other toxic compounds Citation21–39. However, one of the major problems associated with the use of natural products as corrosion inhibitors, is the isolation of the active constituents responsible for the inhibition process. In the light of this, the present study seeks to investigate the possibility of using the fermentation product of Streptomyces griseus (albomycin) as an inhibitor for the corrosion of zinc in 0.01–0.04 M H2SO4. Albomycin is an antibiotics, often used for the treatments of some infections. It can be produced from S. griseus in concentrations of approximately 1 mg/l. The production depends on the phosphate, iron, and ornithine concentrations in the medium. In optimized conditions, the production of albomycin could be increased to 25 mg/l in a feed-batch fermentation. Isolation and purification could be achieved by reversed-phase, size-exclusion chromatography, and preparative high-performance liquid chromatography Citation40. The optimized structure of albomycin is as shown below. From the structure, it is expected that albomycin may be a good inhibitor for the corrosion of zinc in H2SO4 solutions because it has hetero atoms in its structure and contains some functional groups that may facilitate its electron donating ability. Therefore, the objective of the present study is to investigate the adsorption and inhibitive properties of albomycin for the corrosion of zinc in H2SO4
Results and discussions
Effect of concentration of H2SO4/albomycin
shows the variation of weight loss of zinc with time for the corrosion of zinc in 0.01 M H2SO4 containing various concentrations of albomycin at 303 K. From , it is evident that the weight loss of zinc increases with increase in the period of contact but decreases with increase in the concentration of albomycin. Also weight loss of zinc for the blank solution is higher than those obtained for solutions of H2SO4 containing various concentrations of albomycin. Therefore, albomycin is an adsorption inhibitor for the corrosion of zinc in H2SO4 solutions. At higher temperatures and concentrations of H2SO4 (plots not shown), weight loss of zinc was also found to increases with increase in temperature but decreases with increase in the concentration of H2SO4. This indicates that the rate of corrosion of zinc increases with increase in temperature and with increasing concentration of H2SO4. To further assert the above findings, the inhibition efficiencies of various concentrations of albomycin in H2SO4 solutions and the corrosion rates of zinc in similar media were calculated. The results of the calculations are presented in . From the results obtained, the corrosion rates of zinc were found to increase with increasing temperature and with increase in the concentration of H2SO4 but decreases with increase in the concentrations of albomycin. Hence, albomycin retarded the corrosion of zinc in H2SO4 solutions. On the other hand, at a given concentration of H2SO4, the inhibition efficiency of albomycin increases with increasing concentration but decreases with increasing temperature implying that albomycin is an adsorption inhibitor for the corrosion of zinc in H2SO4 and that the adsorption of albomycin on zinc surface supports the mechanism of physical adsorption.
Figure 1. Variation of weight loss with time for the corrosion of zinc in 0.01 M tetraoxosulphate (VI) acid containing various concentration of albomycin at 303 K.
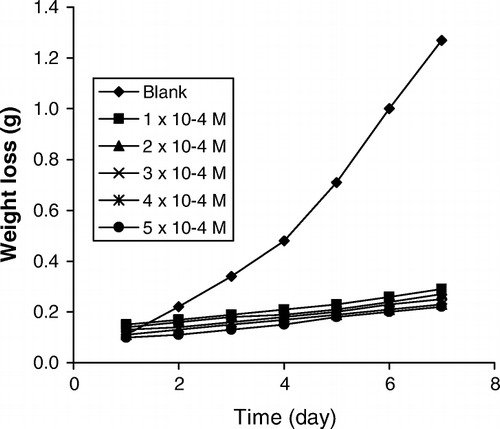
Table 1. Corrosion rate (in gcm−2 h−1) of zinc and inhibition efficiencies (%) of albomycin in various concentrations of H2SO4.
Effect of temperature
The Arrhenius equation was used to calculate the activation energy for the corrosion of zinc in H2SO4 solutions. The concept of Arrhenius theory is provoked by the fact that the corrosion of zinc in H2SO4 requires a minimum energy input and that the corrosion reaction can not proceed until the energy barrier is overcome. From the Arrhenius equation, if the corrosion rate of zinc varies with temperature, then we have the following relation Citation41 Citation42:
Figure 2. Variation of log(CR) with 1/T for the inhibition of zinc corrosion [in (a) 0.01 M; (b) 0.02 M; (c) 0.03 M; and (d) 0.04 M H2SO4] by albomycin.
![Figure 2. Variation of log(CR) with 1/T for the inhibition of zinc corrosion [in (a) 0.01 M; (b) 0.02 M; (c) 0.03 M; and (d) 0.04 M H2SO4] by albomycin.](/cms/asset/b2ec37e2-9ae7-4ded-8df4-f0f2b623305f/tgcl_a_486771_o_f0002g.gif)
Table 2. Activation energies and some thermodynamic parameters for the inhibition of the corrosion of zinc by various concentrations of albomycin.
On the other hands, results obtained from gasometric experiments were 62.45%, 73.34%, 81.01%, 82.35%, and 86.34% for albomycin concentrations of 0.0001 M, 0.0002 M, 0.0003 M, 0.0004 M, and 0.0005 M, respectively. These results are comparable to those obtained from gravimetric experiments.
Thermodynamic/adsorption considerations
Thermodynamic principles can be used to predict the feasibility of a chemical reaction such as that of corrosion. In order to explore these principles, the transition state equation (which can be written as Equation (Equation3)) was adopted Citation43
Figure 3. Variation of log (CR/T) with 1/T for the inhibition of zinc corrosion [in (a) 0.01 M; (b) 0.02 M; (c) 0.03 M; and (d) 0.04 M H2SO4] by albomycin.
![Figure 3. Variation of log (CR/T) with 1/T for the inhibition of zinc corrosion [in (a) 0.01 M; (b) 0.02 M; (c) 0.03 M; and (d) 0.04 M H2SO4] by albomycin.](/cms/asset/05e2c31e-d1ec-4510-9176-ed6c89871139/tgcl_a_486771_o_f0003g.gif)
The relationship between the amount of albomycin adsorbed per unit area of zinc surface and the concentration of albomycin is given by adsorption isotherm. Values of degrees of surface coverage obtained from Equation (Equation8) were used to fit curves for different adsorption isotherms. The tests indicated that the adsorption of albomycin on zinc surface is best described by Langmuir adsorption isotherm. The validity of Langmuir adsorption isotherm can be expressed as follows Citation44
Figure 4. Langmuir isotherm for the adsorption of albomycin on zinc surface in various concentrations of H2SO4 at (a) 303 K; (b) 313 K; and (c) 323 K.
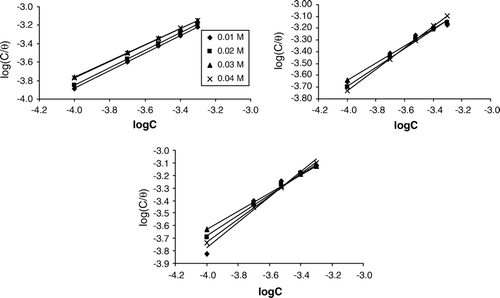
Table 3. Langmuir adsorption parameters and free energies of adsorption of albomycin on the surface of zinc.
The equilibrium constant of adsorption (indicated in Equation (Equation5) above) is related to the free energy of adsorption as follows Citation45
Experimental
Materials
The material used for the study was zinc sheet of composition (w/%); Pb (0.001), Fe (0.002), Cd (0.001), Cu (0.003), and the rest zinc. The sample was mechanically pressed cut into different coupons, each of dimension, 5×4×0.11 cm. Each coupon was degreased by washing with ethanol, dipped in acetone, and allowed to dry in the air before they were preserved in a desiccator. All reagents used for the study were Analar grade and double distilled water was used for their preparation.
The inhibitor (albomycin) was supplied by LIVEMOORE Pharmaceuticals, Ikot Ekpene, Akwa Ibom State, Nigeria and was used without further purification. The concentration range used for the inhibitor was 0.0001–0.0005 M.
Gravimetric method
In the weight loss experiment, the pre-cleaned zinc coupon was dipped in 200 ml of the test solution maintained at 303 K in a thermostated bath. The weight loss was determined by retrieving the coupons at 24 hours interval progressively for 168 hours (7 days). Prior to measurement, each coupon was washed in 5% chromic acid solution (containing 1% silver nitrate) and rinsed in deionized water Citation46. The difference in weight was taken as the weight loss of zinc. The experiments were also carried at 313 and 323 K as described above.
From the weight loss measurements, the inhibition efficiency (%I) of the inhibitor, degree of surface coverage (θ), and the corrosion rate (CR) of zinc were calculated using Equations (Equation3), (Equation8), and (Equation9), respectively Citation46.
Gasometric method
Gasometric experiments were carried out at 303 K using a gasometer, as described in the literature Citation47. The gasometric experiments were conducted at 303 K for the blank (3 M H2SO4) and for H2SO4 solutions containing various concentrations of albomycin. From the volume of hydrogen gas evolved per minute, inhibition efficiencies of various concentrations of the inhibitor were calculated using Equation (Equation10)
Conclusion
Albomycin is a good inhibitor for the corrosion of zinc in H2SO4. The inhibitory action of albomycin is affected by the period of contact with the acid, concentration of the acid/inhibitor, and temperature. Therefore, the performance of albomycin as an inhibitor can be optimized by taking advantage of time, concentration, and temperature. Also thermodynamic and adsorption models can adequately be used to predict the feasibility of the inhibitor.
References
- Abdallah , M. 2004 . Guar Gum as Corrosion Inhibitor for Carbon Steel in Sulphuric Acid Solutions . Port. Electrochim. Acta , 22 : 161 – 175 .
- Abdallah , M. 2004 . Antibacterial Drugs as Corrosion Inhibitors for Corrosion of Aluminium in HCl Solution . Corros. Sci. , 46 : 1981 – 1996 .
- Eddy , N.O. , Ibok , U.J. and Ebenso , E.E. 2010 . Adsorption, Synergistic Inhibitive Effect and Quantum Chemical Studies on Ampicillin and Halides for the Corrosion of Mild Steel . J. Appl. Electrochem. , 40 : 445 – 456 .
- Eddy , N.O. , Ibok , U.J. , Ebenso , E.E. , El Nemr , A. and El Ashry , E.H. 2009 . Quantum Chemical Study of the Inhibition of the Corrosion of Mild Steel in H2SO4 by Some Antibiotics . J. Mol. Mod. , 15 ( 9 ) : 1085 – 1092 .
- Eddy , N.O. ; Ebenso , E.E. Quantum Chemical Studies on the Inhibition Potentials of Some Penicillin Compounds for the Corrosion of Mild Steel in 0.1 M HCl . J. Mol. Model . 2010 . doi: DOI: 10.1007/S00894-0090635-6
- Acharya , S. and Upadhyay , S.N. 2004 . The Inhibition of Corrosion of Mild Steel by Some Flouroquinolones in Sodium Chloride Solution . Trans. Indian Inst. Met. , 57 ( 3 ) : 297 – 306 .
- Eddy , N.O. Theoretical Study on Some Amino Acids and their Potential Activity as Corrosion Inhibitors for Mild Steel in HCl . Mol. Simul . 2010 . doi: DOI: 10.1080/08927020903483270
- Yurt , A. , Balabam , A. , Kandemer , S.U. , Bereket , G. and Erk , B. 2004 . Investigation of Some Schiff Bases as HCl Corrosion Inhibitors for Carbon Steel . Mat. Chem. Phys. , 85 : 420 – 426 .
- Emregul , K.C. , Duzgun , E. and Atakiol , O. 2006 . The Application of Some Polydentate Shiff Base Compounds Containing Aminic Nitrogens as Corrosion Inhibitors for Mild Steel in Acidic Media . Corros. Sci. , 48 : 3243 – 3260 .
- Sheatty , D.S. , Shetty , P. and Nayak , H.V.S. 2006 . Inhibition of Mild Steel Corrosion in Acidic Medium by N-(2-thiophenyl)-N-phenyl thiourea . J. Chidean Chem. Soc. , 51 ( 2 ) : 849 – 853 .
- Ashassi-Sorkhabi , H. , Majidi , M.R. and Seyyedi , K. 2004 . Investigation of Inhibitive Action of Amino Acids Against Steel Corrosion in HCl Solution . Appl. Surf. Sci. , 225 : 176 – 185 .
- Babi-Samardzija , K. , Khaled , K.E. and Hackerman , N. 2005 . N-heterocyclic Amines and Derivatives as Corrosion Inhibitors for Iron in Perchloric Acid . Anti-Corros. Meth. Mater. , 52 ( 1 ) : 11 – 21 .
- Aramaki , K. and Hackermann , N. 1969 . Inhibition Mechanism of Medium Sized Polymethyleneimine . J. Electrochem. Soc. , 2 : 116 – 569 .
- Ita , B.I. 2003 . Inhibition of the Corrosion of Zinc in Tetraoxosulphate (VI) Acid by Phenyl Derivatives of Semicarbazone . J. Nigerian Environ. Soc. , 1 ( 1 ) : 113 – 120 .
- Rajappa , S.K. , Venkatesha , T.V. and Praveen , B.M. 2008 . Chemical Treatment of Zinc Sulphate and its Corrosion Inhibition Studies . Bull. Mat. Sci. , 31 ( 1 ) : 37 – 41 .
- Odoemelam , S.A. , Ogoko , E.C. , Ita , B.I. and Eddy , N.O. 2009 . Inhibition of the Corrosion of Zinc in H2SO4 by 9-deoxy-9a-aza9a-methyl-9a-homoerythromycin A (azithromycin) . Port. Electrochim. Acta , 27 ( 1 ) : 57 – 68 .
- Eddy , N.O. and Odoemelam , S.A. 2008 . Sparfloxacin and Norfloxacin as Corrosion Inhibitors for Mild Steel: Kinetics, Thermodynamics and Adsorption Consideration . J. Mater. Sci. , 4 ( 1 ) : 1 – 5 .
- Eddy , N.O. , Odoemelam , S.A. and Odiongenyi , A.O. 2009 . Joint Effect of Halides and Ethanol Extract of Lasianthera Africana on the Inhibition of the Corrosion of Mild Steel in H2SO4 . J. Appl. Electrochem. , 39 ( 6 ) : 849 – 857 .
- Eddy , N.O. and Odoemelam , S.A. 2009 . Inhibition of the Corrosion of Mild Steel in H2SO4 by Ethanol Extract of Aloe vera . Resin Pigm. Technol. , 38 ( 2 ) : 111 – 115 .
- Awad , M.I. 2006 . Ecofriendly Corrosion Inhibitors: Inhibitive Action of Quinine for Corrosion of Low Carbon Steel in 1 M HCl . J. Appl. Electrochem. , 36 : 1163 – 1168 .
- Kliskic , M. , Radoservic , J. , Gudic , S. and Katalinic , V. 2000 . Aqueous Extract of Rosmmarius officinalis L. as Inhibitor of Al–Mg Alloy Corrosion in Chloride Solution . J. Appl. Electrochem. , 30 : 823 – 830 .
- Rajendran , S. , Ganga , S.V. , Arockiaselvi , J. and Amalraj , A.J. 2005 . Corrosion Inhibition by Plant Extracts – An Overview . Bull. Electrochem. , 21 ( 8 ) : 367 – 377 .
- Okafor , P.C. , Osabor , V. and Ebenso , E.E. 2007 . Eco Friendly Corrosion Inhibitors: Inhibitive Action of Ethanol Extracts of Garcinia Kola for the Corrosion of Aluminium in Acidic Medium . Pigm. Resin Technol. , 36 ( 5 ) : 299 – 305 .
- Umoren , S.A. and Ebenso , E.E. 2008 . Studies of Anti-Corrosive Effect of Raphia hookeri Exudates Gum – Halide Mixtures for Aluminium Corrosion in Acidic Medium . Pigm. Resin Technol. , 37 ( 3 ) : 173 – 182 .
- Moren , S.A. , Obot , I.B. , Ebenso , E.E. and Okafor , P.C. 2008 . Eco-friendly Inhibitors from Naturally Occurring Exudate Gums for Aluminium Corrosion Inhibition in Acidic Medium . Port. Electrochim. Acta , 26 : 267 – 282 .
- Umoren , S.A. and Ebenso , E.E. 2008 . Studies of Anti-Corrosive Effect of Raphia hookeri Exudates Gum–Halide Mixtures for Aluminium Corrosion in Acidic Medium . Pigm. Resin Technol. , 37 : 173 – 182 .
- Sethuran , M.G. and Raja , P.B. 2005 . Corrosion Inhibition of Mild Steel by Datura Metel in Acidic Medium . Pigm. Resin Technol. , 34 ( 6 ) : 327 – 331 .
- Ebenso , E.E. 2003 . Synergistic Effect of Halides Ions on the Corrosion Inhibition of Aluminium in H2SO4 Using 2-Acetylphenothiazine . Mater. Chem. Phys. , 79 : 58 – 70 .
- El-Etre , A.Y. 2006 . Khillah Extract as Inhibitor for Acid Corrosion of SX 316 Steel . Appl. Surf. Sci. , 252 : 8521 – 8525 .
- El-Etre , A.Y. 2003 . Inhibition of Aluminium Corrosion Using Opuntia Extract . Corros. Sci. , 45 : 2485 – 2495 .
- El-Etre , A.Y. 1998 . Natural Honey as Corrosion Inhibitor for Metals and Alloy Copper in Neutral Aqueous Solution . Corros. Sci. , 40 ( 11 ) : 1845 – 1850 .
- Chetounani , A. , Hammouti , B. and Benkaddour , M. 2004 . Corrosion Inhibition of Iron in Hydrochloric Acid Solution by Jojoba Oil . Pigm. Resin Technol. , 33 ( 1 ) : 26 – 31 .
- Bendahou , M.A. , Benadellah , M.B.E. and Hammouti , B.B. 2006 . Study of Rosemary Oil as a Green Corrosion Inhibitor for Steel in 2M H3PO4 . Pigm. Resin Technol. , 35 ( 2 ) : 95 – 100 .
- Bouyanzer , A. and Hammouti , B. 2004 . A Study of Anti-Corrosion Effects of Artemisia Oil on Steel . Pigm. Resin Technol. , 33 ( 5 ) : 287 – 292 .
- Eddy , N.O. 2009 . Ethanol Extract of Phyllanthus Amarus as a Green Inhibitor for the Corrosion of Mild Steel in H2SO4 . Port. Electrochim. Acta , 27 ( 5 ) : 579 – 589 .
- Odiongenyi , A.O. , Odoemelam , S.A. and Eddy , N.O. 2009 . Corrosion Inhibition and Adsorption Properties of Ethanol Extract of Vernonia Amygdalina for the Corrosion of Mild Steel in H2SO4 . Port. Electrochim. Acta , 27 ( 1 ) : 33 – 45 .
- Abiola , O.K. , Oforka , N.C. , Ebenso , E.E. and Nwinuka , N.M. 2007 . Eco-friendly Corrosion Inhibitors: Inhibitive Action of Delonix regia Extract for the Corrosion of Aluminium in Acidic Medium . Anti-Corros. Meth. Mater. , 54 ( 4 ) : 219 – 224 .
- Eddy , N.O. , Odoemelam , S.A. and Ibiam , N. 2009 . Adsorption and Inhibitive Properties of Ethanol Extract of Costus afer on the Inhibition of the Corrosion of Mild Steel in H2SO4 . J. Surf. Sci. Technol. , 25 ( 3–4 ) : 1 – 14 .
- Eddy , N.O. 2009 . Inhibitive and Adsorption Properties of Ethanol Extract of Colocasia esculenta Leaves for the Corrosion of Mild Steel in H2SO4 . Int. J. Phys. Sci. , 4 ( 4 ) : 165 – 171 .
- Pramanik , A. and Braun , V. 2006 . Albomycin Uptake via a Ferric Hydroxamate Transport of Streptococcus pneumoniae R6 . J. Bacteriol. , 118 ( 1 ) : 3878 – 3886 .
- Agrawal , Y.K. , Talati , J.D. , Shah , M.D. , Desai , M.N. and Shah , N.K. 2003 . Schiff Bases of Ethylenediamine as Corrosion Inhibitors of Zinc in Sulphuric Acid . Corros. Sci. , 46 : 633 – 651 .
- Ebenso , E.E. , Ekpe , U.J. , Umoren , S.A. , Jackson , E. , Abiola , O.K. and Oforka , N.C. 2005 . Synergistic Effect of Halides Ions on the Corrosion Inhibition of Aluminium in Acidic Medium by Some Polymers . J. Appl. Polym. Sci. , 100 : 2889 – 2894 .
- Okafor , P.C. and Ebenso , E.E. 2007 . Inhibitive Action of Carica papaya Extracts on the Corrosion of Mild Steel in Acidic Media and their Adsorption Characteristics . Pigm. Resin Technol. , 36 ( 3 ) : 134 – 140 .
- Ekop , A.S. and Eddy , N.O. 2009 . Inhibition of the Corrosion of Mild Steel by Orphnadrine . Aust. J. Basic Appl. Sci. , 2 ( 4 ) : 1258 – 1263 .
- Ita , B.I. and Offiong , O.E. 1995 . The Inhibition of Mild Steel Corrosion in Hydrochloric Acid by 2,2′-Pyridil and α-Pyridoin . Mater. Chem. Phys. , 51 : 203 – 210 .
- Onochukwu , A.I. and Adamu , A.A. 1990 . The Kinetics and Mechanism of Hydrogen Evolution on Corroding Aluminium in Alkaline Medium . Mat. Chem. Phys. , 25 : 227 – 235 .
- Ebenso , E.E. , Eddy , N.O. and Odiongenyi , A.O. 2009 . Inhibition of the Corrosion of Mild Steel by Methocarbamol . Port. Electrochim. Acta , 27 ( 1 ) : 13 – 22 .