Abstract
Polyethylene glycol-600 was used as an efficient and recyclable solvent for the one-pot three component condensation reactions of aryl alkyl ketones, sulfur, and morpholine to produce the corresponding thiomorpholide. This protocol has advantages of high yields, short reaction times, mild reaction conditions, minimal environmental pollution, and simple work up procedure.
Introduction
Thioamides are of importance in medicinal chemistry Citation1 due to their biological activity, against bacterial infection Citation2, as fungicides Citation3, herbicides Citation4, and activators of L-asparaginase Citation5. Apart from these applications, Thioamides are known to be versatile intermediates in organic synthesis Citation6. Particularly in the field of peptide chemistry Citation7, as well as building blocks for the synthesis of five and six membered heterocycles Citation8–11. A traditional approach to their synthesis is the Willgerodt–Kindler reaction which has found only limited application because of the high reaction temperatures and long reaction periods required and the low to moderate yields obtained Citation12–14.The synthesis of thiomorpholide has also been carried out using microwave Citation15 Citation16, but this method requires special reaction instruments and conditions. In recent years, though ionic liquids (ILs) have been found to be effective solvents to improve the Willgerodt–Kindler reaction with good result Citation17, the limitation of the use of ILs as solvents is that the cost is usually expensive and they are difficult to use on an industrial scale Citation18.
Green chemistry is one of the important focuses within organic synthesis, thus it attracts more scientists and researchers to this area. Polyethylene glycol (PEG), which is considered a benign medium due to low vapor pressure, non-flammability, the ease of work up, the ability to act as phase transfer catalysts, good reaction medium, inexpensive price, and eco-friendly for nature Citation19–22, seems to be a perfect solvent alternative to volatile organic solvents and ILs. In fact, PEG has had universal uses in organic reactions for a long time Citation23 Citation24. Various kinds of research have been reported that use PEG as the solvent or medium for organic chemistry Citation25–28. As a part of our on-going research program directed toward the development of new and rapid synthetic methods for the construction of biologically active structural motifs, we intended to develop a rapid, efficient, economic, and easy to scale-up method for the synthesis of thiomorpholide using PEG-600 as green reaction media ().
Results and discussion
In the initial studies, the reaction of acetophenone (2 mmol), sulfur (2 mmol), and morpholine (2 mmol) was performed in different solvents without any added catalyst to synthesize the compound 1-Morpholin-4-yl-2-phenyl-ethanethione (4a). It was observed that among the tested solvents (, Entries 4–7), the reaction (, Entry 4) in PEG-600 was more facile and proceeded to give best yields (96%) when the reaction mixture was stirred at 100°C for 1.2 hours. Moreover, there are many potential advantages of replacing these volatile or toxic organic solvents with PEG-600. Therefore, PEG-600 is an optimal reaction medium for the reaction.
Table 1. Synthesis of thiomorpholide using PEG-600 as green reaction media.
The effect of temperature was also been studied by carrying out the model reaction of 1-Morpholin-4-yl-2-phenyl-ethanethione (4a) in PEG-600 at different temperatures. As shown in (Entries 1–2), the reaction did proceed when the reaction temperature was 35°C or 50°C. However, the obtained yield remained low even after longer reaction time (24 h). However, at elevated temperature (75–100°C) using PEG-600 gave better results in terms of yield and reaction time. Hence, the conditions of Entry 4, shown in , were the optimized reaction conditions.
In order to evaluate the generality of the process, we studied the reaction of various aryl alkyl ketones with morpholine and sulfur in PEG-600 at 100°C. The results are presented in . Both electron rich as well as electron deficient acetophenones reacted well with morpholine in the presence of sulfur to give thioamides in excellent yields. Bulky and sterically hindered substrates such as 2-hydroxyacetophenone, 2-acetylnaphthalene, and 2-aminoacetophenone reacted smoothly under similar conditions to produce the corresponding thioamides (, Entries g, i, and j). In the case of halogenated acetophenones, no side products were observed arising from nucleophilic displacement of halogen by morpholine under these conditions (, Entry b).
Table 2. Synthesis of thiomorpholide using PEG-600 as green reaction media.
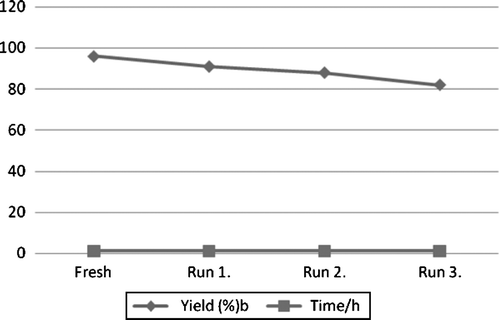
In the present procedure, PEG-600 acts as a clean solvent. Moreover, PEG-600 is a recyclable reagent. In the reaction of 1-Morpholin-4-yl-2-phenyl-ethanethione (4a) (), we recycled PEG-600 three times and the reaction proceeded cleanly with good yields, 91%, 88%, and 82% (, Run 1, 2, and 3), respectively. Slight weight loss of PEG-600 was observed from cycle to cycle due to mechanical loss. The present methodology offers very attractive features such as excellent yields, simple reaction conditions, and easy work up. Moreover, the use of PEG-600 as reaction medium makes this process a green synthesis.
Figure 1. Recycling yields.
aReaction condition: acetophenone (2 mmol), sulfur (2 mmol), and morpholine (2 mmol); solvent PEG-600; 100°C.
bIsolated and unoptimized yields.
In the classical Willgerodt–Kindler reaction, morpholine was generally used in large excess as a solvent, as well as a reactant Citation12 Citation15. The use of PEG-600 as a solvent minimizes the quantity of morpholine in the reaction. This is because of the activation of ketones by PEG-600. The main advantage of this procedure is that high conversions (79–96%) were achieved in short reaction times (1.2–2.4 h) by using PEG-600.
Experimental
General remarks
All chemicals were purchased from Sigma–Aldrich and Lancaster and were used as such. All reactions and purity of thiomorpholide compounds (4a–k) were monitored by thin layer chromatography (TLC) using aluminum plates coated with silica gel (Merck) using 20% ethyl acetate and 80% hexane as eluent. IR spectra were recorded on Perkin–Elmer FTIR-1710 spectrophotometer using Nujol film. 1H NMR spectra were recorded on a Bruker Avance Spectrospin 300 (300 MHz) using TMS as internal standard and chemical shift are in ▵. GC–MS mass spectra were recorded on a Waters LCT Micromass. The temperature of the reaction mixture was measured through a non-contact infrared thermometer (AZ, Mini Gun type, Model 8868).
General procedure for the synthesis of thiomorpholide
In a 50 ml round bottom flask, acetophenone (2 mmol), sulfur (2 mmol), and morpholine (2 mmol) in PEG-600 (1 ml) were mixed and stirred at 100°C for an appropriate time (), then treated with cool water. The solid product (4a), which separated out, was filtered, washed with water and dried. The crude product was recrystallized from ethanol. The progress of the reaction was monitored by TLC using hexane-ethyl acetate (8:2). In the recycled reaction, after isolation of the product from the reaction system, the mother liquor, which is the mixture of PEG-600 and water, extracted with ether (PEG being insoluble in ether). The ether layer was decanted, and mother liquor dried for 4 h under the infrared light or distilled directly to eliminate water. The next run was performed using the same conditions.
Selected physical and spectral data
1-Morpholin-4-yl-2-phenyl-ethanethione (4a)
Yellow solid, m.p.: 60–65°C, IR (KBr): υmax: 3474, 3023, 2966, 2860, 1964, 1710, 1485, 1274, 1104, 958, 705, and 624 cm−1. 1H NMR (300 MHz, CDCl3): ▵ 7.17–7.22 (m, 5H), 4.30–4.35 (t, 2H), 4.30 (s, 2H), 3.70–3.78 (t, 2H), 3.55–3.52 (t, 2H), and 3.30–3.40 (t, 2H); ES-MS E/Z 221 (M+).
2-(4-Bromo-phenyl)-1-morpholin-4-yl-ethanethione (4b)
Light yellow solid, m.p.: 92–96°C, IR (KBr): υmax: 3414, 2921, 2857, 1485, 1434, 1271, 1217, 1110, 1032, 960, 724, and 612 cm−1. 1H NMR (300 MHz, CDCl3): ▵ 7.40–7.50 (d, J=7.49 Hz, 2H), 7.20 (d, J=6.50 Hz, 2H), 4.35 (t, 2H), 4.22 (s, 2H), 3.70–3.80 (t, 2H), 3.60 (t, 2H), and 3.40 (t, 2H); ES-MS E/Z 300 (M+).
Conclusion
In summary, we report a novel, mild, and highly efficient protocol for the three-component condensation of aryl alkyl ketones, sulfur, and morpholine to produce thiomorpholides in PEG-600. The present method has some notable advantages compared to the previous methods such as high conversions, operational simplicity, enhanced reaction rates, cleaner reaction profiles, and ease of isolation of products, which makes the process potentially useful for industrial applications for the synthesis of thiomorpholides. We indicated that PEG-600 can replace traditional organic solvents and some ILs in the reaction. PEG-600 is a favorable solvent which sets a good example of green chemistry.
Acknowledgements
The authors are thankful to The Director, School of Chemical Sciences, Swami Ramanand Teerth Marathwada University, Vishnupuri, Nanded for providing laboratory facilities.
References
- Smith , J. ; Liras , J.L. ; Schneider , S.E. ; Anslyn , E.V. J. Org. Chem . 1996 , 61 , 8811 8818
- Mallams , A.K. ; Morton , J.B. ; Reichert , P.J. J. Chem. Soc. Perkin Trans . 1981 , 1 , 2186 2208 .
- Schroeder , D.C. Chem. Rev . 1955 , 55 , 181 228 .
- Sarkis , G.Y. ; Faisal , E.D. J. Heterocycl. Chem . 1985 , 22 , 137 140 .
- Bandgar , B.P. ; Gawande , S.S. ; Warangkar , S.C. ; Totre , J.V. Bioorg. Med. Chem . 2010 , 18 , 3618 3624
- Metzner , P. Synthesis 1992 , 12 , 1185 1199 .
- Weinstein , B. , Chemistry and Biochemistry of Amino Acids, Peptides and Proteins: a survey of recent developments , Marcel Dekker Inc., New York , 1983 ; Vol. 7 , pp. 267 357 .
- Jagodzinski , S.T. Chem. Rev . 2003 , 103 , 197 227 .
- Griffin , T.S. ; Woods , T.S. ; Klayman , D.L. Advances in Heterocyclic Chemistry ; Katritzky , A.R. , Boulton , A.J. , New York : Academic Press , 1975 ; Vol. 18 , pp 99 .
- Moghaddam , F.M. ; Boinee , H.Z. Tetrahed. Lett . 2003 , 44 , 6253 6255 .
- Moghaddam , F.M. ; Boinee , H.Z. Tetrahedron 2004 , 60 , 6085 6089 .
- Brown , E.V. Synthesis 1975 , 358 373 , and references cited therein .
- Sheldon , R. J. Chem. Soc. Chem. Commun . 2001 , 2399 2407 .
- Gordon , C.M. Appl. Catal. A: Gen. 2001 , 222 , 101 117 .
- Nooshabadi , M. ; Aghapoor , K. ; Darabi , H.R. ; Mojtahedi , M.M. Tetrahed. Lett . 1999 , 44 , 7549 7552 .
- Moghaddam , F.M. ; Hojabri , L. ; Dohendou , M. Synth. Commun . 2003 , 24 , 4279 4284 .
- Yadav , J.S. ; Reddy , B.V.S. ; Kondaji , G. ; Reddy , J.S.S. ; Nagaiah , K. J. Mol. Catal. A: Chem . 2007 , 266 , 249 253 .
- Laus , G. ; Bentivoglio , G. ; Schottenberger , H. ; Kahlenberg , V. ; Kopacka , H. ; Röder , T. ; Sixta , H. Lenzinger Berichte 2005 , 84 , 71 85 .
- Harris , J.M. ; Hundley , N.H. ; Shannon , T.G. ; Evelyn , C. J. Org. Chem . 1982 , 47 , 4789 4791 .
- Baj , S. ; Siewniak , A. Appl. Catal. A: Gen . 2007 , 321 , 175 179 .
- Heldebrant , D.J. ; Jessop , P.G. J. Am. Chem. Soc . 2003 , 125 , 5600 5601 .
- Chen , J. ; Spear , S.K. ; Huddleston , J.G. ; Rogers , R.D. Green Chem . 2005 , 7 , 64 82 .
- Kleber , C. ; Andrade , Z. ; Alves , L.M. Curr. Org. Chem . 2005 , 9 , 195 218 .
- Feng , B. ; Hu , Y. ; Li , H. ; Hou , Z.S. Chin. J. Org. Chem . 2008 , 28 , 381 389 .
- Kidwai , M. ; Bhatnagar , D. ; Mishra , N.K. ; Bansal , V. Catal. Commun . 2008 , 9 , 2547 2549 .
- Bandgar , B.P. ; Patil , S.A. ; Korbad , B.L. ; Bandgar , S.B. ; Hote , B.S. Aust. J. Chem . 2008 , 61 7 , 552 555 .
- Bandgar , B.P. ; Patil , S.A. ; Korbad , B.L. ; Bandgar , S.B. ; Hote , B.S. ; Chavan , V. Aust. J. Chem . 2008 , 61 9 , 700 703 .
- Das , B. ; Krishnaiah , M. ; Balasubramanyam , P. ; Veeranjaneyulu , B. ; Kumar , D.N. Tetrahed. Lett . 2008 , 49 , 2225 2227 .