Abstract
A new protocol for Biginelli (or like) reaction has been developed under solvent-free grinding method using catalytic amount of hydrated ferric nitrate or clayfen. The advantage of this novel protocol lies in the avoidance of organic solvent, high yield, energy efficiency, variation of substrates, and use of inexpensive catalyst. The recycling of catalyst is also possible with clayfen for more than three times. Furthermore, the catalytic activity of ferric nitrate retains its efficiency in methanol and acetone as reaction medium.
1. Introduction
In recent years, the growing interest on exploitation of multicomponent reactions (MCR) Citation1–4 for the fast development of library of biologically active compounds is a promising greener route in terms of higher atom economy as compared to multistep reaction. One such MCR is the classical Biginelli Citation5 three-component reaction, which involves acid catalyzed one pot condensation of an aldehyde, a β-ketoester, and urea in ethanol for the synthesis of 3,4-dihydropyrimidinone derivatives (DHPM). A 3,4-DHPM derivatives exhibit excellent pharmacological and therapeutic properties such as antibacterial, antitumour, and antiinflammatory activities as well as behaving as calcium Citation6–8 channel blockers, α-1a-antagonists, and neuropeptides Y (NPY) antagonists. For the Biginelli reaction, a large number of new techniques Citation9–14 such as microwave-assisted synthesis, ultrasound irradiation, solvent-free condition, etc. and various Lewis acid catalysts Citation15–19 such as BF3OEt2, InBr3, LaCl3.H2O, Yb(OTf)3, CuCl2, etc. were used to improve this conversion. Despite their potential utility, all these methods were limited to aromatic aldehydes, open chain β-dicarbonyl compound, and urea or thiourea. Very recently, the novel Biginelli like reaction Citation20–24 has been utilized to synthesize DHPM derivatives using various types of carbonyl compound such as acetophenone, cyclic ketone, cyclic-β-diketones, β-ketolactones, etc. However, many of these methods are low yields, very long reaction times, harsh reaction condition, and use of toxic solvent. Thus it becomes necessary to adopt experimental conditions which remove all these limitations to obtain a better yield of product under greener technique. Toda and his group Citation25 shown that many reactions such as Grignard reaction, Reformatsky reaction, and Aldol condensation can be conducted in high yield by just grinding solids together using mortar and pestle. Furthermore, these solid-state grinding reactions (or solvent-free reaction) have several advantages: more eco-friendly, low costs, higher yields, and simplicity in process and handling which are mainly important from the point of view of industrial manufacturing.
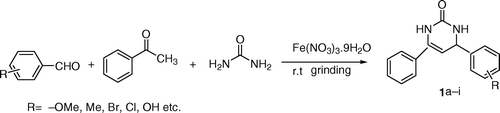
In continuation of our earlier work Citation26–28 on greener paths, we report herein, an efficient Biginelli (or like) reaction by using inexpensive Fe (NO3)3.9H2O as catalyst to synthesize large number of Biginelli products including 5-unsubstituted 3,4-dihydropyrimidinone 1 and 5-substituted 3,4-dihydropyrimidin-2(1H)-one 2 under solvent-free mechano-chemical mixing method using mortar and pestle.
2. Results and discussion
We first started to investigate the reaction () of anisaldehyde (1 mmol), acetophenone (1 mmol), and urea (1.5 mmol) in presence of different catalysts (0.1 mmol) () under solvent-free grinding method and in presence of various solvents. From these results, it was observed that except hydrated ferric nitrate and clayfen, all others catalysts were found to be inactive for this reaction. The hydrated ferric nitrate took less reaction time (, Entry 8) as compared (, Entry 9) to supported catalyst (clayfen). Furthermore, the catalyst ferric nitrate retained its activity in methanol and acetone as reaction medium (, Entries 14, 15).
Table 1. Synthesis of 5-unsubstituted 3,4-dihydropyrimidinone 1 using 0.1 mmol of different catalysts.
The above optimized condition of the Biginelli like reaction of acetophenone under solvent-free method, extended to other aromatic aldehydes with different substituents and the results are summarized in , Entries 1–9. All aromatic aldehydes containing different substituent reacted efficiently to form the corresponding 5-unsubstituted 3,4-dihydropyrimidinone 1 derivatives.
Table 2. Synthesis of 5-unsubstituted 3,4-dihydropyrimidinone 1 and 5-substituted 3,4dihydropyrimidinone 2 derivatives.
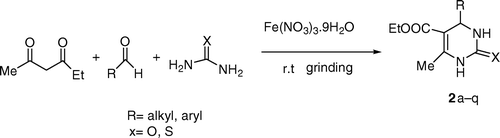
Finally, we applied this method for the synthesis of 5-substituted 3,4-dihydropyrimidone derivatives 2 using acetoacetic ester () as carbonyl compound () with different aldehydes and urea (or thiourea).
In our overall study on the different types of experimentations to come up with a simple and environmentally benign reaction system, we explored efficacy of ferric nitrate in synthesizing more importantly 5-unsubstituted 3,4-dihydropyrimidinone 1 compounds employing green method. However, problem regarding the regeneration of the catalyst is a hindrance in assigning it as green catalyst. This problem can also be solved by using clay supported ferric nitrate (clayfen) as catalyst. Although, the catalytic activity of clayfen is less than ferric nitrate, it can be easily regenerated by altering the aqueous work up with hot ethanol solvent. The insoluble clayfen residue washed several times with hot ethanol and dried in a vacuum desicator. The regenerated clayfen can be reused three times without appreciable loss of catalytic activity.
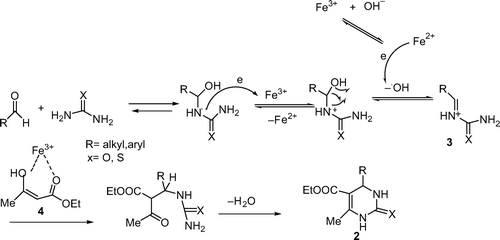
The good performance of hydrated iron (III) nitrate as catalyst may be ascribed to its easy electron accepting property as a strong oxidant which catalyzes the formation of iminium intermediate 3 in the slowest step as well as activating β-ketoester 4 in the process of Biginelli reaction based on the proposed mechanism by Kappe Citation29 shown in .
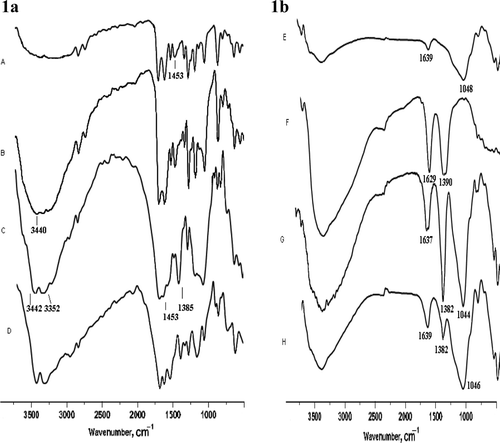
The progress of these solid-state reactions was further monitored by measurement of the IR spectrum of the reaction mixture of acetophenone, anisaldehyde, and urea () at various time intervals (a) using clayfen as catalyst where gradual change of N–H and C = O stretching frequencies represents the completion of reaction into Biginelli product. The IR spectra of regenerated clayfen shown similar absorptions peak with clayfen catalyst (b).
Figure 1. (a) IR spectra of reaction mixture (acetophenone, anisaldehyde, and urea) at various time intervals, 60 mins (A), 120 mins (B), 3 hr (C), and pure product (D) where region 3000–3500 cm−1 represents splitting of νN–H stretching into two peaks and region 1500–1700 cm−1 shows gradual change of νC = O stretching frequencies. (b) IR spectra of Monmorillonite K-10 (E), Fe (NO3)3.9H2O (F), clayfen (G), and regenerated clayfen (H).
We have developed a general and efficient greener path for the synthesis of 5-unsubstituted 3,4-dihydropyrimidinone 1 and 5-substituted 3,4-dihydropyrimidinone 2 under solvent-free grinding condition using hydrated ferric nitrate/clayfen as catalyst. The advantage of this novel protocol lies in the avoidance of organic solvent, high yield, energy efficiency, variation of substrates, and cheaper catalyst.
3. Experimental
3.1. General
All reactions were monitored by TLC using silica gel (Merck, 60–120 mesh). 1HNMR spectra were recorded on a Varian 400 MHz FT-NMR spectrometer using CdCl3 as solvent and TMS as internal standard. The elements analyses were performed on a Perkin–Elmer 20 Analyzer. IR data were recorded at Nicolet instruments 410-FTIR spectrophotometer using KBr optics. All products were characterized by comparison of their IR, 1HNMR, and mass spectra with those of authentic samples Citation20–24 Citation30–35 . The starting chemicals were obtained from commercial suppliers and used without further purification.
3.2. General method for the synthesis of Biginelli (or like) product under solvent-free grinding technique
A mixture of acetophenone (1 mmol), aldehyde (1 mmol), urea (1.5 mmol), and hydrated ferric nitrate (or clayfen) (0.1 mmol) was gently ground by hand using mortar and pestle of appropriate size. The progress of the reaction was monitored by TLC which indicates the formation of single product. The mixture becomes a sticky paste during the course of reaction which finally solidifies on completion of reaction. Finally, it was washed with a cold saturated solution of NaHCO3 (5 ml) and then filtered through a sintered funnel to afford the crude product which was further purified by recrystallization (ethanol). With clayfen catalyst, the reaction mixture was dissolved in hot ethanol and filtered. The insoluble clayfen residue washed several times with hot ethanol and dried in a vacuum desicator for reuse.
3.3. Spectral data of selected compounds
3.3.1. 4-(4-Chlorophenyl) 3,4-dihydro-6-phenylpyrimidin-2(1H)-one 1b
Melting point 266°C; 1HNMR (400 MHz, DMSO-d 6); δ 8.67 (s, 1H, NH), 8.10 (s, 1H, NH), 7.52–7.30 m, (m, 9H, Ar–H), 5.46(d, J=2.8 Hz, 1H, CH), 5.16 (d, 1H, J=2.8 Hz, CH); IR (KBr) cm−1 3230, 2934, 1682, 1575, and 1467; CHN Anal. Cal. C16H13ClN2O; C 67.48, H 4. 56, N 9.84; Found C 66.75, H 4.55, N 10.00.
3.3.2. 3,4-Dihydro-4-(4-methoxyphenyl)-6-phenylpyrimidin-2(1H)-one 1c
Melting point 258°C; 1HNMR (400 MHz, DMSO-d 6); δ 12.00 (s, 1H, NH), 9.30 (s, 1H, NH), 8.35–7.28 (m, 9H, Ar–H), 6.94 (d, J=8.7 Hz, 1H, CH), 5.42 (d, 1H, J=8.7 Hz, CH), 3.76 (s, 3H, OCH3); IR (KBr) cm−1 3384, 2935, 1615, 1520, 1410; CHN Anal. Cal. C17H16N2O2; C 72.85, H 5.71, N 10.00; Found C 72.60, H 5.73, N 10.10.
3.3.3. 3,4-Dihydro-4-(4-hydroxyphenyl)-6-phenylpyrimidin-2(1H)-one 1f
Melting point 256°C; 1HNMR (400 MHz, DMSO-d 6); δ 9.22 (s, 1H, NH), 8.16–7.54 (m, 9H, Ar–H), 7.36–7.32 (s, 1H, NH), 7.25 (d, J=8.8 Hz, 1H, CH), 5.52 (s, 1H, OH) 5.12 (d, 1H, J=8.8 Hz, CH); IR (KBr) cm−1 3387, 2920, 1628, 1517, and 1448; CHN Anal. Cal. C16H14N2O2; C 72.17, H 5.26, N 10.50; Found C 72.30, H 5.33, N 10.40.
3.3.4. 3, 4-Dihydro 4-(3, 4-dimethoxyphenyl)-6-phenylpyrimidin-2(1H)-one 1i
Melting point 244°C; 1HNMR (400 MHz, DMSO-d 6); δ 11.92 (s, 1H, NH), 8.55 (s, 1H, NH), 8.52–7.50 (m, 8H, Ar–H), 7.45 (d, J=8.3 Hz, 1H, CH), 7.08 (d, 1H, J=8.3Hz, CH), 3.90 (s, 3H, OCH3), 3.88 (s, 3H, OCH3); IR (KBr) cm−1 3277, 2930, 1617, 1515, and 1462; CHN Anal. Cal. C18H18N2O3; C 69.67, H 5.85, N, 9.03; Found C 69.60, H 5.78, N 9.00.
Acknowledgements
The authors are thankful to Council of Scientific and Industrial Research, New Delhi, India for granting a Research Project No. 01(2067)/06/EMR-II to RB and CIFC, IIT Guwahati for their co-operation in sample analysis.
References
- Plunkett , M. ; Ellman , J. Combinatorial chemistry New Drugs Sci. Am . 1997 , 276 , 68 73 .
- Schreiber , S.L. Science 2000 , 287 , 1964 1969 .
- Kappe , C.O. Acc. Chem. Res . 2000 , 33 , 879 888 .
- Weber , L. ; Illgen , K. ; Almstetter , M. Synlett 1999 , 3 , 366 374 .
- Biginelli , P. Gazz. Chim. Ital . 1893 , 23 , 360 416 .
- (a) Kappe , C.O. Eur. J. Med. Chem . 2000 , 35 , 1043 ; (b) Kappe, C.O. Molecules 1998, 3, 1–9.
- Atwal , K.S. ; Swanson , B.N. ; Unger , S.E. ; Floyd , D.M. ; Moreland , S. ; Hedberg , A. ; O'Reilly , B.C. J. Med. Chem . 1991 , 34 , 806 811 .
- Rovnyak , G.C. ; Kimball , S.D. ; Beyer , B. ; Cucinotta , G. ; Dimarco , J.D. ; Gougoutas , J. ; Hedberg , A. ; Malley , M. ; McCarthy , J.P. ; Zhang , R.A. ; Morreland , S. J. Med. Chem . 1995 , 38 , 119 129 .
- Stadler , A. ; Kappe , C.O. J. Chem. Soc. Perkin. Trans . 2 . 2000 , 1363 1368 .
- Al-Mousawi , S.M. ; El-Apasery , M.A. ; Elnagdi , M.H. Molecules 2010 , 15 , 58 67 .
- Desai , B. ; Dallinger , D. ; Kappe , C.O. Tetrahedron 2006 , 62 , 4651 4644 .
- Yadav , J.S. ; Reddy , B.V.S. ; Reddy , K.B. ; Raj , K.S. ; Prasad , A.R. J. Chem. Soc. Perkin. Trans. I. 2001 , 1939 1941 .
- Azizian , J. ; Mohammadi , A.A. ; Karimi , A.R. ; Mohammadizadeh , M.R. App. Cat. A: Gen . 2006 , 300 , 85 88 .
- Hong , M. ; Cai , C. J. Heterocy. Chem . 2009 , 46 , 1430 1432 .
- Fu , N-Y. ; Yuan , Y-F. ; Cao , Z. ; Wang , S-W. ; Wang , J-T. ; Peppe , C. Tetrahedron 2002 , 58 , 4801 4807 .
- Hu , E.H. ; Sidler , D.R. ; Dolling , U-H. J. Org. Chem . 1998 , 63 , 3454 3457 .
- Lu , J. ; Bai , Y. ; Wang , Z. ; Yang , B. ; Ma , H. Tetrahed. Lett . 2000 , 41 , 9075 9078 .
- Ma , Y. ; Qian , C. ; Wang , L. ; Yang , M. J. Org. Chem . 2000 , 65 , 3864 3868
- Mirza-Aghayan , M. ; Moradi , A. ; Bolourtchian , M. ; Boukheroub , R. Syn. Commun . 2010 , 40 , 8 20 .
- Wang , Z-T. ; Xu , L-W. ; Xia , C-G. ; Wang , H-Q. Tetrahed. Lett . 2004 , 45 , 7951 1953 .
- Liang , B. ; Wang , X. ; Wang , J-X. ; Du , Z. Tetrahedron 2007 , 63 , 1981 1986 .
- Saini , A. ; Kumar , S. ; Sandhu , J.S. Indian J. Chem. 46B . 2007 , 1690 1694 .
- Shaabani , A. ; Sarvary , A. ; Rahmati , A. ; Rezayan , A.H. Lett. Org. Chem . 2007 , 4 , 68 71 .
- Shen , Z-L. ; Xu , X-P. ; Ji , S-J. J. Org. Chem . 2010 , 75 , 1162 1167 .
- Tanaka , K. ; Toda , F. Chem. Rev . 2000 , 100 , 1025 1074 .
- (a) Phukan , M. ; Borah , K.J. ; Borah , R. Syn. Commun . 2008 , 38 , 3068 3073 ; (b) Phukan, M.; Borah, K.J.; Borah, R. Green Chem. Lett. Rev. 2009, 2, 249–253 .
- Borah , K.J. ; Phukan , M. ; Borah , R. Syn. Commun . 2008 , 38 , 3082 3087 .
- Thakur , A.J. ; Borah , R. Syn. Commun . 2007 , 37 , 933 939 .
- Kappe , C.O. J. Org. Chem . 1997 , 62 , 7201 7204 .
- Lu , J. ; Bai , Y. Synthesis 2002 , 4 , 466 469 .
- Kappe , C.O. ; Wagner , U.G. Heterocycles 1989 , 29 , 761 769 .
- Kappe , C.O. ; Kumar , D. ; Varma , R.S. Synthesis 1999 , 1799 1803 .
- Heravi , M.M. ; Bakhtiari , K. ; Bamoharram , F.F. Cat. Commun . 2006 , 7 , 373 376 .
- Gholap , A.R. ; Venkatesan , K. ; Daniel , T. ; Lahoti , R.J. ; Srinivasan , K.V. Green Chem . 2004 , 6 , 147 150 .
- Esfahani , M.N. ; Khosropour , A.R. Bull. Korean Chem. Soc . 2005 , 26 , 1331 1332 .