Abstract
The ability of the bis(allyl)-ruthenium(IV) complex [{RuCl(µ-Cl)(η3: η3-C10H16)}2] (C10H16=2,7-dimethylocta-2,6-diene-1,8-diyl) to promote intermolecular [2+2+2] alkyne cyclotrimerization reactions in aqueous media under microwave (MW) irradiation has been evaluated. Advantages and disadvantages of using MW vs. conventional thermal heating are discussed.
Introduction
Environmental concerns in laboratories and chemical industries are increasingly recognized, and concepts such as the E-factor Citation1, atom economy Citation2 Citation3, and green chemistry Citation4–9 are nowadays considered as essential driving forces in the development of sustainable chemical processes. In this sense, a crucial factor in realizing a “green” process involves the choice of a safe, non-toxic, eco-friendly, and cheap solvent Citation10–13. Water is undoubtedly one of the most appealing candidates. Thereby, the development of organic transformations in water has become one of the major cornerstones in modern chemistry, with a wide variety of highly efficient and selective synthetic protocols conducted in aqueous media being already available for practical uses Citation14–23.
Although generally neglected by chemists until recently, another important aspect to reduce the environmental impact of a synthetic procedure is the optimization of its energy consumption Citation4–9. In this context, the use of microwave (MW) irradiation represents a convenient alternative to the conventional thermal heating since a more effective energy transfer to the system takes place. This fact results in an extremely rapid warming, thus shortening considerably the reaction times Citation24–27. The satisfactory application of MWs in a large number of organic transformations and metal-catalyzed reactions, mostly carried out in organic media using MW-transparent solvents such as toluene, THF, or dichloromethane, attests to the usefulness of this technique in synthesis Citation28–30. Furthermore, in order to seek “greener” synthetic protocols, the combined use of MW irradiation, as a non-classical low-energy-consuming heating source, and water, as an environmentally friendly solvent, to perform organic reactions has recently emerged as a promising new field of research that is waiting to be explored in depth Citation31–33.
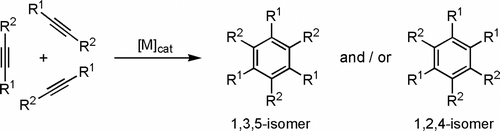
On the other hand, transition-metal-catalyzed inter- or intramolecular [2+2+2] cyclotrimerization of alkynes is one of the most powerful and elegant synthetic tools presently available for the construction of substituted arenes (). Thus, after the pioneering work of Reppe in 1948 Citation34, a wide variety of metal complexes have been developed for this atom economical transformation and relevant applications in the synthesis of challenging molecules, including some natural products, have been disclosed Citation35–42.
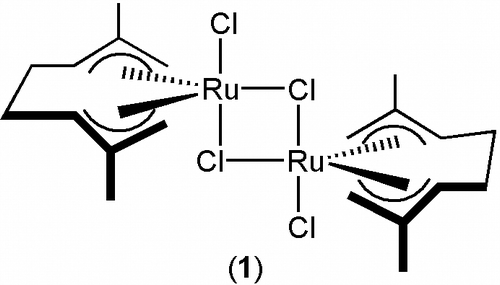
With most of these cyclotrimerization reactions being performed in organic media, the discovery of transition-metal complexes active in water is highly desirable. However, efforts in this direction have been scarce and only a very limited number of efficient and selective catalysts in this medium have seen the light to date Citation43 Citation44. Among them, the commercially available (Strem Chemicals Inc.) bis(allyl)-ruthenium(IV) dimer [{RuCl(µ-Cl)(η3:η3-C10H16)}2] (C10H16=2,7-dimethylocta-2,6-diene-1,8-diyl; 1 in ) Citation45 merits to be highlighted since, as recently described by us Citation46, it displays an outstanding performance in the intermolecular cyclization of both terminal and internal alkynes, and a wide tolerance to functional groups Citation47.
In order to assess whether a change in the heating method (MW vs. thermal) could affect the course of these catalytic reactions, we decided to explore the behavior of complex 1 under MW irradiation. It must be noted that, while MW-assisted metal-mediated alkyne cyclotrimerization process in organic media are known Citation48–52, to the best of our knowledge, no examples in water have been previously described Citation53.
Results and discussion
In order to find optimal reaction conditions, exploratory studies were conducted using the cyclotrimerization of diethyl acetylenedicarboxylate (2a) into hexaethyl mellitate (3a) as model reaction (see ). Conditions A employed in our previous work Citation46, i.e. 0.2 M solution of the alkyne in a mixture H2O/MeOH (90:10 v/v) and 2.5 mol% of complex 1, led to the aromatic product 3a in 88% GC-yield after 14 h of conventional oil-bath heating at 75°C (entry 1). Gratifyingly, we have now found that performing the same reaction under controlled MW heating at 75°C (conditions B) results in the chemoselective and almost quantitative formation of 3a (99% GC-yield) after only 5 h (entry 2), thus demonstrating the enhancing effects of MW irradiation on this Ru-catalyzed reaction.
Table 1. Catalytic cyclotrimerization of dialkyl acetylenedicarboxylates 2a–b in aqueous medium using complex [{RuCl(µ-Cl)(η3:η3-C10H16)}2] (1).a
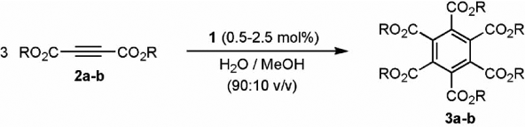
More importantly, the rate of this cyclotrimerization process can be dramatically accelerated, without sacrificing the chemoselectivity, by application of successive short (1 min) irradiations using a constant MW power of 300 W (T max=155°C, P max=100 psi). Under this new conditions C, diethyl acetylenedicarboxylate (2a) is completely converted into hexaethyl mellitate (3a) after only 15 min of irradiation (entry 3). Subsequent purification by column chromatography on silica gel provided an analytically pure sample of 3a in an excellent 91% isolated yield. It is also noteworthy that application of conditions C allows the reduction of the catalyst loading. Thus, as shown in entry 4, using only 0.5 mol% of dimer 1, quantitative conversion of 2a into 3a could be attained within 1 h. A rapid and selective transformation of dimethyl acetylenedicarboxylate (2b) into hexamethyl mellitate (3b) was also observed under conditions C using a catalyst loading of 2.5 mol% (99% GC-yield after 5 min; entry 6), making them much more appealing than the classical thermal ones (entry 5). Thus, all subsequent [2+2+2] cyclotrimerization reactions were performed with a catalyst loading of 2.5 mol% under constant 300 W MW irradiation.
The results obtained with various terminal alkynes confirmed the scope of this cyclization reaction (see ), the use of MWs (even entries) leading in all cases to a significant decrease in reaction time compared to that required under standard thermal conditions (odd entries). Both aromatic (2c–f) and aliphatic (2g–k) terminal alkynes, as well as the acetylenic esters 2l–m or the keto-alkyne 2n, could be smoothly cyclotrimerized within 1 h, the reactions delivering the corresponding arenes in 71–92% isolated yield after silica-gel chromatography. However, we must note that in a few cases (entries 8, 12, 16, and 18) formation of minor amounts of methyl-ketones as by-products was observed, as the result of a competing Ru-catalyzed Markovnikov hydration of the alkyne Citation54 Citation55. Such a side reaction does not occur using thermal heating, conditions in which the only competing process observed was the eventual dimerization of the substrates (entries 13, 15, and 17).
Table 2. Catalytic cyclotrimerization of terminal alkynes 2c–n in aqueous medium using complex [{RuCl(µ-Cl)(η3:η3-C10H16)}2] (1).a
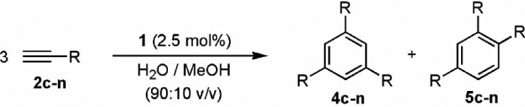
Interestingly, the use of MWs does not affect the regioselectivity of the process, the reactions leading to mixtures of regioisomers in very similar ratios to those observed under conventional thermal heating. As expected on the basis of steric grounds, formation of the symmetric 1,3,5-substituted isomers 4 was in almost all cases favored with respect to the more highly strained 1,2,4-substituted products 5.
Experimental section
All the alkynes 2a–n employed in this study were obtained from commercial suppliers and used as received. The bis(allyl)-ruthenium(II) complex [{RuCl(µ-Cl)(η3:η3C10H16)}2] (1) was prepared by following the method reported in the literature Citation56. NMR spectra were recorded on a Bruker DPX300 instrument at 300 MHz (1H) or 75.4 MHz (13C) using SiMe4 as standard. GC/MS measurements were performed on a Agilent 6890N equipment coupled to a 5973 mass detector (70 eV electron impact ionization) using a HP-1MS column.
General procedure for the MW-assisted cyclotrimerization reactions
Under nitrogen atmosphere, a pressure-resistant septum-sealed glass vial was charged with the corresponding alkyne 2a–n (1 mmol), the catalyst [{RuCl(µ-Cl)(η3:η3-C10H16)}2] (1) (15 mg, 2.5 mmol; 5 mol% of Ru), a magnetic stirring bar, water (4.5 ml) and methanol (0.5 ml). The vial was then placed inside the cavity of a CEM Discover® S-Class MW synthesizer and exposed to successive short MW irradiations (1 min) using a constant irradiation power of 300 W (T max=155°C, P max=100 psi; cooling to 60°C between each irradiation). The progress of the reaction was monitored by regular sampling and analysis by gas chromatography. Once the reaction finished, the vial was cooled to room temperature and the organic product extracted with diethyl ether (3×10 ml). After drying with anhydrous MgSO4, the solvent was evaporated under vacuum, and the crude residue purified by flash chromatography over silica gel using EtOAc/hexane (1:10) as eluent. The identity of the resulting arenes was assessed by 1H and 13C{1H} NMR spectroscopy and GC/MSD. 3a Citation57: White solid; 1H NMR (CDCI3) δ 1.25 (t, 18H, J hh =7.0 Hz, CH3), 4.24 (q, 12H, 3 J hh =7.0 Hz, CH2); 13C{1H} NMR (CDCl3) δ 14.2 (s, CH3), 63.1 (s, CH2), 134.2 (s, Carom), 165.21 (s, CO); MS (EI 70 eV) m/z 510 (5%, M+), 465 (40), 420 (20), 391 (50), 363 (40), 335 (30), 307 (20), 289 (100). 3b Citation58: White solid; 1H NMR (CDCl3) δ 3.82 (s, 18H, CH3); 13C{1H} NMR (CDCl3) δ 53.96 (s, CH3), 134.37 (s, Carom), 165.59 (s, CO); MS (EI 70 eV) m/z 426 (5%, M+), 395 (100), 364 (10), 349 (15), 293 (10), 248 (5). 4c Citation59: White solid; 1H NMR (CDCl3) δ 7.10–8.00 (m, 18H, CHarom); 13C{1H} NMR (CDCl3) δ 128.0, 128.6, 128.9, and 129.5 (s, CHarom), 141.8 and 143.0 (s, Carom); MS (EI 70 eV) m/z 306 (100%, M+), 289 (25), 276 (10), 228 (15). 5c Citation59: White solid; 1H NMR (CDCl3) δ 7.07–7.92 (m, 18H, CHarom); 13C{1H} NMR (CDCl3) δ 125.8, 126.8, 127.1, 127.2, 127.3, 127.8, 128.2, 129.0, 129.1, 129.2, 130.3, and 130.5 (s, CHarom), 139.3, 140.2, 141.0, 141.2, 141.6, and 142.1 (s, Carom); MS (EI 70 eV) m/z 306 (100%, M+), 289 (15), 276 (5), 228 (15). 4d Citation59: Yellow solid; 1H NMR (CDCl3) δ 7.05–8.20 (m, 15H, CHarom); 13C{1H} NMR (CDCl3) δ 129.0, 129.1, and 129.2 (s, CHarom), 133.9, 139.7, and 142.0 (s, Carom); MS (EI 70 eV) m/z 408 (100%, M+), 372 (20), 338 (80), 302 (60). 5d Citation59: Yellow solid; 1H NMR (CDCl3) δ 7.07–8.24 (m, 15H, CHarom); 13C{1H} NMR (CDCl3) δ 128.0, 128.2, 128.4, 128.8, 128.9, 129.1, 129.3, 129.4, and 129.6 (s, CHarom), 130.5, 131.6, 132.5, 137.0, 138.5, 138.7, 139.2, 139.6, and 141.3 (s, Carom); MS (EI 70 eV) m/z 408 (100%, M+), 372 (10), 338 (20), 302 (30). 4e (59): Yellow solid; 1H NMR (CDCl3) δ 7.04–7.80 (m, 15H, CHarom); 13C{1H} NMR (CDCl3) δ 131.0, 131.2, and 133.2 (s, CHarom), 122.1, 139.5, and 141.4 (s, Carom); MS (EI 70 eV) m/z 542 (100%, M+), 462(10), 382 (80), 302 (80), 276 (20). 5e (59): Yellow solid; 1H NMR (CDCl3) δ 7.07–7.76 (m, 15H, CHarom); 13C{1H} NMR (CDCl3) δ 124.5, 125.9, 127.5, 128.2, 128.5, 130.8, 131.0, 131.4, and 131.6 (s, CHarom), 121.3, 121.4, 122.0, 137.8, 138.5, 139.0, 139.5, 139.7, and 139.8 (s, Carom); MS (EI 70 eV) m/z 542 (100%, M+), 462 (5), 382 (10), 302 (50), 276 (10). 4f Citation59: White solid; 1H NMR (CDCl3) δ 2.42 (s, 9H, CH3), 7.16–7.98 (m, 15H, CHarom); 13C{1H} NMR (CDCl3) δ 21.8 (s, CH3), 127.8, 129.3, and 130.2 (s, CHarom), 137.9, 139.1, and 142.8 (s, Carom); MS (EI 70 eV) m/z 348 (100%, M+), 333 (10), 318 (10), 303 (5). 5f Citation59: White solid; 1H NMR (CDCl3) δ 2.44, 2.50, and 2.52 (s, 3H each, CH3), 7.15–7.96 (m, 15H, CHarom); 13C{1H} NMR (CDCl3) δ 22.0, 22.1, and 22.2 (s, CH3), 125.2, 127.0, 127.6, 129.5, 129.6, 129.8, 129.9, 130.3, and 130.4 (s, CHarom), 136.6, 136.7, 137.7, 138.4, 138.9, 139.4, 139.8, 140.7, and 141.5 (s, Carom); MS (EI 70 eV) m/z 348 (100%, M+), 333 (5), 318 (5), 303 (5). 4g Citation60: Yellow oil; 1H NMR (CDCl3) δ 0.95 (m, 9H, CH3), 1.37 (m, 6H, CH2), 1.56 (m, 6H, CH2), 2.58 (m, 6H, CH2), 6.83 (s, 3H, CHarom); 13C{1H} NMR (CDCl3) δ 14.7 (s,CH3), 23.2, 34.5, and 36.4 (s, CH2), 126.5 (s, CHarom), 143.3 (s, Carom); MS (EI 70 eV) m/z 246 (50%, M+), 217 (10), 204 (100), 189 (10), 161 (30), 147 (60). 5g Citation60: Yellow oil; 1H NMR (CDCl3) δ 0.95 (m, 9H, CH3), 1.35 (m, 6H, CH2), 1.55 (m, 6H, CH2), 2.56 (m, 6H, CH2), 6.97 (m, 2H, CHarom), 7.07 (d, 1H, 3 J hh =7.5 Hz, CHarom); 13C{1H} NMR (CDCl3) δ 14.3, 14.5, and 14.6 (s, CH3), 23.2, 23.5, 23.6, 31.0, 31.6, 33.1, 34.3, 36.0, and 38.0 (s, CH2), 126.4, 129.6, and 129.9 (s, CHarom), 140.8, 140.9, and 141.0 (s, Carom); MS (EI 70 eV) m/z 246 (30%, M+), 217 (5), 204 (30), 189 (5), 161 (100), 147 (25). 4h Citation61 : Yellow oil; 1H NMR (CDCl3) δ 0.91 (m, 9H, CH3), 1.21–1.48 (m, 18H, CH2), 1.56 (m, 6H, CH2), 2.56 (m, 6H, CH2), 6.82 (s, 3H, CHarom); 13C{1H} NMR (CDCl3) δ 14.0 (s, CH3), 22.5, 29.0, 31.3, 31.5, and 31.7 (s, CH2), 125.7 (s, CHarom), 142.6 (s, Carom); MS (EI 70 eV) m/z 330 (50%, M+), 301 (5), 287 (10), 273 (5), 260 (100), 245 (5), 189 (30). 5h Citation61: Yellow oil; 1H NMR (CDCl3) δ 0.93 (m, 9H, CH3), 1.21–1.48 (m, 18H, CH2), 1.55 (m, 6H, CH2), 2.54 (m, 6H, CH2), 6.96 (m, 2H, CHarom), 7.06 (d, 1H, 3 J hh =7.7 Hz, CHarom); 13C{1H} NMR (CDCl3) δ 13.9 (br, CH3), 22.5, 28.0, 28.4, 28.5, 28.7, 28.8, 29.4, 31.6, 32.3, 32.7, 35.6, 35.9, and 37.5 (s, CH2), 125.6, 128.8, and 129.1 (s, CHarom), 140.0, 140.2, and 140.4 (s, Carom); MS (EI 70 eV) m/z 330 (60%, M+), 260 (30), 245 (5), 189 (100). 4i Citation62: Yellow oil; 1H NMR (CDCl3) δ 0.91 (d, 18H, 3 J hh =6.3 Hz, CH3), 1.86 (m, 3H, CH(CH3)2), 2.44 (d, 6H, 3 J hh =6.2 Hz, CH2), 6.76 (s, 3H, CHarom); 13C{1H} NMR (CDCl3) δ 24.4 (s,CH3), 32.3 (s, CH(CH3)2), 47.4 (s, CH2), 129.4 (s, CHarom), 142.9 (s, Carom); MS (EI 70 eV) m/z 246 (30%, M+), 203 (100), 163 (10), 147 (20). 5i: Yellow oil; 1H NMR (CDCl3) δ 0.88–0.95 (m, 18H, CH3), 2.04 (m, 3H, CH(CH3)2), 2.40–2.51 (m, 6H, CH2), 6.89 (s, 1H, CHarom), 6.91 and 7.02 (d, 1H each 3 J hh =7.7 Hz, CHarom); 13C{1H} NMR (CDCl3) δ 23.8, 24.5, and 24.6 (s, CH3), 30.2, 31.7, and 31.8 (s, CH(CH3)2), 43.9, 47.1, and 50.8 (s, CH2), 129.5, 128.2, and 131.7 (s, CHarom), 132.9,133.7, and 136.7 (s, Carom); MS (EI 70 eV) m/z 246 (40%, M+), 20 3 (100), 161 (60), 145 (15). 4j: White solid; 1H NMR (CDCl3) δ 1.88–2.04 (m, 6H, CH2), 2.57–2.71 (m, 12H, CH2), 7.08–7.32 (m, 18H, CHarom); 13C{1H} NMR (CDCl3) δ 29.7, 33.0, and 35.6 (s, CH2), 125.7, 128.3, and 128.5 (s, CHarom), 139.7 and 142.2 (s, Carom); MS (EI 70 eV) m/z 432 (40%, M+), 328 (20), 224 (20), 120 (60), 91 (100). 5j: White solid; 1H NMR (CDCl3) δ 1.88–2.04 (m, 6H, CH2), 2.57–2.71 (m, 12H, CH2), 7.08–7.32 (m, 18H, CHarom); 13C{1H} NMR (CDCl3) δ 31.8, 32.3, 32.9, 35.1, 35.5, 35.9, and 36.0 (s, CH2), 125.7, 125.9, 126.1, 128.3, 128.5, 129.1, and 129.3 (s, CHarom), 137.4, 139.9, 142.3, and 142.4 (s, Carom); MS (EI 70 eV) m/z 432 (60%, M+), 328 (10), 224 (20), 120 (20), 91 (100). 4k: Yellow oil; 1H NMR (CDCl3) δ 0.89–1.66 (m, 30H, CH2), 2.01 (m, 3H, CH), 2.39 (m, 6H, CH2), 6.71 (s, 3H, CHarom); 13C{1H} NMR (CDCl3) δ 26.5, 29.6, 33.4, and 44.0 (s, CH2), 39.4 (s, CH), 127.3 (s, CHarom), 140.5 (s, Carom); MS (EI 70 eV) m/z 366 (60%, M+), 284 (100), 201 (20), 119 (60). 5k: Yellow oil; 1H NMR (CDCl3) δ 0.89–1.66 (m, 30H, CH2), 2.19 (m, 3H, CH), 2.41 (m, 6H, CH2), 6.84 (s, 1H, CHarom), 6.86 and 6.71 (d, 1H each, 3 J hh =7.3 Hz, CHarom); 13C{1H} NMR (CDCl3) δ 26.1, 26.3, 26.4, 32.6, 33.1, 40.3, 40.7, and 43.7 (s, CH2), 39.8 (br, CH), 126.0, 129.7, and 131.0 (s, CHarom), 136.4, 137.9, and 138.8 (s, Carom); MS (EI 70 eV) m/z 366 (80%, M+), 284 (30), 201 (100), 119 (50). 4l Citation63: Yellow oil; 1H NMR (CDCl3) δ 3.83 (s, 9H, CH3), 8.72 (s, 3H, CHarom); 13C{1H} NMR (CDCl3) δ 52.4 (s, CH3), 128.7 (s, CHarom), 131.0 (s, Carom), 165.1 (s, CO); MS (EI 70 eV) m/z 252 (5%, M+), 221 (100), 193 (10), 162 (15). 5l Citation63: Yellow oil; 1H NMR (CDCl3) δ 3.85 (s, 6H, CH3), 3.88 (s, 3H, CH3), 7.65 (d, 1H, 3 J hh =8.2 Hz, CHarom), 8.09 (dd, 1H, 3 J hh =8.2 Hz, 4 J hh =1.5 Hz, CHarom), 8.30 (d, 1H, 4 J hh =1.5 Hz, CHarom); 13C{1H} NMR (CDCl3) δ 52.6 and 52.7 (s, CH3), 103.0, 132.1, and 134.3 (s, CHarom), 131.4, 132.3, and 136.0 (s, Carom), 166.6 and 167.4 (s, CO); MS (EI 70 eV) m/z 252 (15%, M+), 221 (100), 193 (15), 162(10). 4m Citation61 : Yellow oil; 1H NMR (CDCl3) δ 1.34 (t, 9H, 3 J hh =7.4 Hz, CH3), 4.37 (q, 6H, 3 J hh =7.4 Hz, CH2), 8.77 (s, 3H, CHarom); 13C{1H} NMR (CDCl3) δ 13.9 (s, CH3), 61.5 (s, CH2), 133.8 (s, CHarom), 136.1 (s, Carom), 164.9 (s, CO); MS (EI 70 eV) m/z 294 (5%, M+), 249 (40), 221 (100), 193 (50), 176 (15), 148 (20). 5m Citation61: Yellow oil; 1H NMR (CDCl3) δ 1.29 (m, 9H, CH3), 4.12 (m, 6H, CH2), 7.69 (d, 1H, 3 J hh =8.0 Hz, CHarom), 8.12 (dd, 1H, 3 J hh =8.0 Hz, 4 J hh =1.7 Hz, CHarom), 8.32 (d, 1H, 4 J hh =1.7 Hz, CHarom); 13C{1H} NMR (CDCl3) δ 13.9, 14.1, and 14.2 (s, CH3), 61.5, 61.7, and 61.8 (s, CH2), 128.3, 129.5, and 131.4 (s, CHarom), 131.3, 131.9, and 132.5 (s, Carom), 164.8, 166.4, and 166.9 (s, CO); MS (EI 70 eV) m/z 294 (10%, M+), 266 (10), 249 (100), 221 (50), 193 (30), 176 (10), 148 (10). 4n Citation64: Colorless oil; 1H NMR (CDCl3) δ 2.72 (s, 9H, CH3), 8.71 (s, 3H, CHarom); 13C{1H} NMR (CDCl3) δ 27.5 (s, CH3), 132.4 (s, CHarom), 138.6 (s, Carom), 197.3 (s, CO); MS (EI 70 eV) m/z 204 (20%, M+), 189 (100), 161 (10), 119 (15).
Conclusions
In summary, we have demonstrated that fast intermolecular [2+2+2] alkyne cyclotrimerization reactions can be performed in aqueous media by employing the commercially available [{RuCl(µ-Cl)(η3:η3-C10H16)}2] catalyst in conjunction with the enhancing effects of MW irradiation. Several terminal and internal alkynes were subjected to these unprecedented conditions delivering the corresponding arenes in good to excellent yields and remarkable short reaction times (<1 h). We believe that this new synthetic protocol will be of interest to a wide range of syntheticFootnote1 organic chemistsFootnote2, who may include itsFootnote3 use in theirFootnote4 future research programs, providing also impetus for further developments in the scarcely explored field of MW-assisted metal-catalyzed reactions in environmentally benign aqueous media Citation31–33.
Acknowledgements
This work was supported by the Spanish MICINN (Projects CTQ2006-08485/BQU, CTQ2009-08746/BQU, and Consolider Ingenio 2010 (CSD2007-00006)) and the Gobierno del Principado de Asturias (FICYT Project IB08-036). J.F. and S.E.G.-G. thank MICINN and the European Social Fund for the award of a Ph.D. grant and a Ramon y Cajal contract, respectively.
Notes
1. For a review on the preparation, reactivity, and catalytic applications of dimer 1, see Citation45
2. Application of complex 1 in the polycyclotrimerization of diynes has also been recently described Citation47.
3. Non-metal-catalyzed intramolecular alkyne cyclotrimerization reactions promoted by focused MW heating in mixtures DMF/H2O have been reported Citation53.
4. The ability of ruthenium complexes to promote Markovnikov hydrations of terminal alkynes is well-documented Citation54 Citation55.
References
- Sheldon , R.A. Chem. Ind. (London) 1992 , 903 906 ; Sheldon, R.A. Pure Appl. Chem. 2000, 72, 1233–1246; Sheldon, R.A. Green Chem. 2007, 9, 1273–1283 .
- Trost , B.M. Science 1991 , 254 , 1471 1477 ; Trost, B.M. Angew. Chem. Int. Ed. Engl.1995, 34, 259–281; Trost, B.M. Acc. Chem. Res. 2002, 35, 695–705 .
- Trost , B.M. , Frederiksen , M.U. and Rudd , M.T. 2005 . Angew. Chem. Int. Ed. , 44 : 6630 – 6666 .
- Anastas , P.T. ; Warner , J.C. Green Chemistry Theory and Practice ; Oxford University Press : Oxford, , UK , 1998 .
- Matlack , A.S. Introduction to Green Chemistry ; Marcel Dekker : New York , 2001 .
- Clark , J.H. ; Macquarrie , D.J. Handbook of Green Chemistry and Technology ; Blackwell Publishing : Abingdon, , UK , 2002 .
- Lancaster , M. Green Chemistry: An Introductory Text ; RSC Editions : Cambridge, , UK , 2002 .
- Poliakoff , M. , Fitzpatrick , J.M. , Farren , T.R. and Anastas , P.T. 2002 . Science , 297 : 807 – 810 .
- Sheldon , R.A. ; Arends , I. ; Hanefeld , U. Green Chemistry and Catalysis ; Wiley-VCH : Weinheim, , Germany , 2007 .
- Nelson , W.M. Green Solvents for Chemistry: Perspectives and Practice ; Oxford University Press : New York , 2003 .
- Clark , J.H. and Taverner , S.J. 2007 . Org. Process Res. Dev. , 11 : 149 – 155 .
- Kerton , F.M. Alternative Solvents for Green Chemistry ; RSC Publishing : Cambridge, , UK , 2009 .
- Cue , B.W. and Zhang , J. 2009 . Green Chem. Lett. Rev. , 2 : 193 – 211 .
- Li , C.-J. Chem. Rev . 1993 , 93 , 2023 2035 ; Li, C.-J. Chem. Rev. 2005, 105, 3095–3166 .
- Lubineau , A. ; Auge , J. ; Queneau , Y. Synthesis 1994 , 741 760 .
- Lindström , U.M. 2002 . Chem. Rev. , 102 : 2751 – 2772 .
- Andrade , C.K.Z. and Alves , L.M. 2005 . Curr. Org. Chem. , 9 : 195 – 218 .
- Li , C.-J. and Chen , L. 2006 . Chem. Soc. Rev. , 35 : 68 – 82 .
- Chen , L. and Li , C.-J. 2006 . Adv. Synth. Catal. , 345 : 1459 – 1484 .
- Herrerías , C.I. , Yao , X. , Li , Z. and Li , C.-J. 2007 . Chem. Rev. , 107 : 2546 – 2562 .
- Li , C.-J. ; Chan , T.H. Comprehensive Organic Reactions in Aqueous Media ; John Wiley & Sons : Hoboken, NJ , 2007 .
- Lindström , U.M. Organic Reactions in Water: Principles, Strategies and Applications ; Blackwell Publishing : Oxford, , UK , 2007 .
- Skouta , R. 2009 . Green Chem. Lett. Rev. , 2 : 121 – 156 .
- Kappe , C.O. Angew. Chem. Int. Ed . 2004 , 43 , 6250 6284 ; Kappe, C.O. Chem. Soc. Rev. 2008, 37, 1127–1139 .
- Gronnow , M.J. , White , R.J. , Clark , J.H. and Macquarrie , D.J. 2005 . Org. Process Res. Dev. , 9 : 516 – 518 .
- Strauss , C.R. 2009 . Aust. J. Chem. , 62 : 3 – 15 .
- Polshettiwar , V. , Nadagouda , M.N. and Varma , R.S. 2009 . Aust. J. Chem. , 62 : 16 – 26 .
- Appukkuttan , P. ; Van der Eycken , E. Eur. J. Org. Chem . 2008 , 1133 1155 .
- Caddick , S. and Fitzmaurice , R. 2009 . Tetrahedron , 65 : 3325 – 3355 .
- Kappe , C.O. ; Dallinger , D. ; Murphree , S.S. Practical Microwave Synthesis for Organic Chemists ; Wiley-VCH : Weinheim, , Germany , 2009 .
- Dallinger , D. and Kappe , C.O. 2007 . Chem. Rev. , 107 : 2563 – 2591 .
- Polshettiwar , V. ; Varma , R.S. Chem. Soc. Rev . 2008 , 37 , 1546 1557 ; Polshettiwar, V.; Varma, R.S. Acc. Chem. Res.2008, 41, 629–639 .
- Polshettiwar , V. ; Varma , R.S. Aqueous Microwave Chemistry ; RSC Publishing : Cambridge, , UK , 2010 .
- Reppe , W. and Sweckendiek , W.J. 1948 . Justus Liebigs Ann. Chem. , 560 : 104 – 116 .
- Vollhardt , K.P.C. 1984 . Angew. Chem. Int. Ed. Engl. , 23 : 539 – 556 .
- Schore , N.E. 1988 . Chem. Rev. , 88 : 1081 – 1119 .
- Lautens , M. , Klute , W. and Tam , W. 1996 . Chem. Rev. , 96 : 49 – 92 .
- Saito , S. and Yamamoto , Y. 2000 . Chem. Rev. , 100 : 2901 – 2915 .
- Chopade , P.R. and Louie , J. 2006 . Adv. Synth. Catal. , 348 : 2307 – 2327 .
- Gandon , V. ; Aubert , C. ; Malacria , M. Chem. Commun . 2006 , 2209 2217
- Shibata , T. and Tsuchikama , K. 2008 . Org. Biomol. Chem. , 6 : 1317 – 1323 .
- Galan , B.R. and Rovis , T. 2009 . Angew. Chem. Int. Ed. , 48 : 2830 – 2834 .
- Cadierno , V. ; Crochet , P. ; Garcia-Garrido , S.E. In Green Chemistry Research Trends ; Pearlman , J.T. Nova Science Publishers : New York , 2009 , pp 97 130 .
- Adriaenssens , L. , Severa , L. , Vávra , J. , Šálová , T. , Hývl , J. , Čížková , M. , Pohl , R. , Šaman , D. and Teplý , F. 2009 . Collect. Czech. Chem. Commun. , 74 : 1023 – 1034 .
- Cadierno , V. , Crochet , P. , García-Garrido , S.E. and Gimeno , J. 2006 . Curr. Org. Chem. , 10 : 165 – 183 .
- Cadierno , V. , Garcia-Garrido , S.E. and Gimeno , J. 2006 . J. Am. Chem. Soc. , 128 : 15094 – 15095 .
- Liu , J. , Lam , J.W.Y. , Jim , C.K.W. , Yue , Y. , Deng , R. , Hong , Y. , Qin , A. , Sung , H.H.Y. , Williams , I.D. , Jia , G. and Tang , B.Z. 2009 . Macromolecules , 42 : 7367 – 7378 .
- Efskind , J. and Undheim , K. 2003 . Tetrahedron Lett. , 44 : 2837 – 2839 .
- Young , D.D. , Sripada , L. and Deiters , A. 2007 . J. Comb. Chem. , 9 : 735 – 738 .
- Shanmugasundaram , M. , Aguirre , A.L. , Leyva , M. , Quan , B. and Martinez , L.E. 2007 . Tetrahedron Lett. , 48 : 7698 – 7701 .
- Sripada , L. , Teske , J.A. and Deiters , A. 2008 . Org. Biomol. Chem. , 6 : 263 – 265 .
- Teske , J.A. and Dieters , A. 2008 . J. Org. Chem. , 73 : 342 – 345 .
- Saaby , S. , Baxendale , I.R. and Ley , S.V. 2005 . Org. Biomol. Chem. , 3 : 3365 – 3368 .
- Alonso , F. , Beletskaya , I.P. and Yus , M. 2004 . Chem. Rev. , 104 : 3079 – 3159 .
- Beller , M. , Seayad , J. , Tillack , A. and Jiao , H. 2004 . Angew. Chem. Int. Ed. , 43 : 3368 – 3398 .
- Salzer , A. , Bauer , A. , Geyser , S. , Podewils , F. , Turpin , G.C. and Ernst , R.D. 2004 . Inorg. Synth. , 34 : 59 – 65 .
- Lindner , E. , Jansen , R.-M. , Mayer , H.A. , Hiller , W. and Fawzi , R. 1989 . Organometallics , 8 : 2355 – 2360 .
- Desroy , N. ; Robert-Peillard , F. ; Toueg , J. ; Hénaut , C. ; Duboc , R. ; Rager , M.-N. ; Savignac M. ; Genêt , J.-P. Synthesis 2004 , 2665 2672 .
- Tagliatesta , P. , Floris , B. , Galloni , P. , Leoni , A. and D'Arcangelo , G. 2003 . Inorg Chem. , 42 : 7701 – 7703 .
- Hilt , G. ; Vogler , T. ; Hess , W. ; Galbiati , F. Chem. Commun . 2005 , 1474 1475 .
- Tanaka , K. , Toyoda , K. , Wada , A. , Shirasaka , K. and Hirano , M. 2005 . Chem. Eur. J. , 11 : 1145 – 1156 .
- Johansson , I. and Olsson , T. 1981 . Acta Chem. Scand. A , 35 : 481 – 488 .
- Herberhold , M. , Yan , H. , Milius , W. and Wrackmeyer , B. 2000 . Organometallics , 19 : 4289 – 4294 .
- Yan , H. , Beatty , A.M. and Fehlner , T.P. 2002 . Organometallics , 21 : 5029 – 5037 .