Abstract
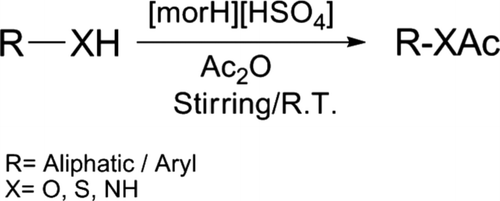
Morpholinium bisulfate [morH][HSO4] found to be highly inexpensive, efficient, and reusable catalyst to promote the acylation of phenols, thiols, alcohols, and amines in presence of acetic anhydride under solvent-free condition with excellent yields by stirring at room temperature. Present methodology deals with remarkable features of mild reaction condition, high yielding, shorter reaction time, easy workup, and more importantly, environmentally benign.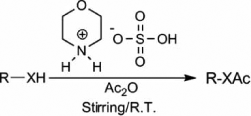
Introduction
When used in place of classical organic solvents, room temperature ionic liquids offer a new and environmentally benign approach toward modern synthetic chemistry Citation1–3. This approach has been successfully applied in several classical organic transformation Citation4 Citation5. Ionic liquids have interesting advantages, such as extremely low vapor pressure, excellent thermal stability, reusability, and the ability to dissolve many organic and inorganic substrates Citation6.
Acylation is a fundamental process in organic chemistry, that provides an efficient route for protecting groups during oxidation, peptide coupling, and glycosidation reactions Citation7 Citation8. Protection of –OH, –SH and –NH2 group is usually achieved by acylation with acetic anhydride, due to the ease of deprotection Citation9 Citation10. The various catalysts developed for acylation include nucleophilic agents Citation11 Citation12 such as DMAP and Bu3P, Lewis acids such as metal halides Citation13–15, metal triflates Citation16–20, metal perchlorates Citation21–26, ionic liquids Citation27, and several solid acids, such as nafion-H Citation28, zeolites Citation29, clays Citation30, HBF4–SiO2 Citation31, HClO4–SiO2 Citation32, zirconocene bis(perfluorooctanesulfonate)s Citation33 , ruthenium(III) chloride Citation34, and alumina supported MoO3 Citation35.
However, industrial applications of some of the above mentioned catalytic systems suffer from limitations, such as non-recoverability, stringent conditions, use of halogenated solvents, and hazardous materials. Also lack of reusability, high catalyst loading, and high cost of some of these reported catalysts determine us think about developing a mild and cost-effective methodology.
As a part of our research work Citation36–38 to develop economical and environment friendly routes in organic transformation, we have tried to implement [morH][HSO4] Citation39 () as a cost-effective and efficient catalyst for the acylation of alcohols, phenols, thiols, and amines at room temperature.
Results and discussion
Herein, we wish to report the acylation of alcohols, thiols, phenols, and amines using acetic anhydride in the presence of catalytic amount of morpholinium bisulfate by stirring at room temperature (). Preparation of morpholinium bisulfate was found to be highly cost effective Citation39.
In order to explore the scope of the methodology, a wide range of structurally diverse and functionalized phenols, thiols, alcohols, and amines that underwent acylation with excellent yield in most of the cases were examined. During the experiment various electron-donating and withdrawing substituents were taken into consideration (). It is noted that [morH][HSO4] not only accelerates the rate of the reaction, but also increases the yield.
Table 1. Acylation of phenols, alcohols, thiols, and amines using [morH][HSO4] as a catalyst.
During acylation, halogenated phenols (, Entries 6, 7, and 12) and nitro-phenols (, Entries 10 and 11) showed some influence on the resultant time and yield which may be due to electronic effect of substituent. Aliphatic secondary alcohols (, Entry 16 and 19) require more time than the primary, which could be due to the steric effect exerted on the chain. This catalytic system also works well with tertiary alcohols (, Entry 20). Same catalytic system also acylates thiols with good yield (, Entries 13 and 14). Rapid acylation of amines was observed during experiments (, Entries 21–25). From results, acylation of amino group was more rapid than hydroxy groups, whereas the acylation of thiols was considerably slower than hydroxy groups.
Subsequently, an attempt has been made for the reusability of [morH][HSO4] considering (, Entry 2) as model reaction. The yields after the catalyst were found to be from 97 to 94%, which was obtained within time periods ranging from 5 to 12 min after 1–5 runs ().
Table 2. Recovery and reusability of catalyst.
From those results, it can be noticed that we have successfully reused the catalyst in subsequent runs and the catalytic activity of catalyst did not show any significant decrease even after five runs.
Experimental
All reagents used were from Merck and Aldrich and used without further purification. IR spectra were recorded on JASCO FT-IR 4100 spectrometer in KBr with absorption in cm–1. 1HNMR was measured on Bruker DPX 400-MHz spectrometer. Mass spectra [ES-MS] were recorded on a Water-Micro mass Quattro-II spectrophotometer.
General procedure for acylation
4-Anisidine (1 mmol) was treated with acetic anhydride (1.5 mmol) under neat condition. Morpholinium bisulfate [morH][HSO4] (0.05 mmol) was added to the reaction mask which was stirred for an appropriate time. The course of the reaction was monitored by TLC. After completion of reaction, the mixture was extracted with ethyl acetate to afford 4-methoxyphenyl acetate which was in full agreement with spectral data.
During acylation of 2-aminothiophenol and ethylene glycol (, Entry 14, and 17), three equivalent amounts of AC2O were used and in case of tert-butanol (, Entry 16), 4.5 equivalent amounts of AC2O were used.
p-Methoxyphenyl acetate
IR (KBr): 1758 (C = O) cm–1; 1H NMR (CDCl3): δ ppm 2.24 (s, 3H, –COCH3), 3.72 (s, 3H, –OCH3), and 6.82–7.03 (m, 4H, Ar–H); MS (m/z): 166 [M+] (, Entry 2).
Ethane-1,2-diyl di-acetate
IR (KBr): 1730 (C = O) cm–1; 1H NMR (CDCl3): δ ppm 2.07 (s, 6H) and 4.25 (s, 4H); MS (m/z): 146 [M+] (, Entry 17).
p-Tolyl-acetamide
IR (KBr): 3301 (–NH) and 1665 (C = O) cm–1; 1H NMR (CDCl3): δ ppm 2.18 (s, 3H, –COCH3), 2.27 (s, 3H, –CH3), and 7.01–7.12 (m, 4H, Ar–H). MS (m/z): 149 [M+] (, Entry 24).
Thioacetic acid S-(2-acetylamino-phenyl)ester
IR (KBr): 3320 (–NH), 1730 (S–COCH3), and 1690 (NH–COCH3) cm–1; 1H NMR (CDCl3): δ ppm 2.12 (s, 3H, –COCH3), 2.40 (s, 3H, –COCH3), 7.11–7.69 (m, 4H, Ar–H), and 7.78 (s, 1H, –NH); MS (m/z): 209 [M+] (, Entry 14)..
t-Butyl acetate
IR (KBr): 1785 (C = O) cm–1, 1H NMR (CDCl3): δ ppm 1.42 (s, 9H, (CH3)3), 2.14 (s, 3H, –OCH3); MS (m/z): 116 [M+] (, Entry 20).
Reusability of catalyst
After completion of reaction, the mixture was extracted with ethyl acetate and placed for a while for the formation of two phases. The aqueous layer of ionic liquid phase was simply separated with a separating funnel, washed with ether, dried under reduced pressure, and directly reused for further analysis.
Conclusion
Introduction of [morH][HSO4] can be considered as an interesting new alternate route for acylation. The merits of this method includes solvent-free, highly inexpensive, ease of handling, easily recycling, and reuse of the catalyst. Therefore, with increasing “green” concern, the solvent-free conditions employed in the present method will make it environment friendly and useful for industrial applications. Further application of [morH][HSO4] for other reaction systems are under investigation.
Acknowledgements
The authors are thankful to The Head, Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India, for providing laboratory facilities.
References
- Welton , T. 1999 . Chem. Rev. , 90 : 2071 – 2083 .
- Sheldon , R. 2001 . Chem. Commun. , 23 : 2399 – 2407 .
- Holbrey , J.D. and Seddon , K.R. 1999 . Clean Prod. Process , 1 : 223 – 236 .
- Cole , A.C. , Jensen , J.L. and Ntai , I. 2002 . J. Am. Chem. Soc. , 124 : 5962 – 5963 .
- Morrison , D.W. , Forbes , D.C. and Davis , J.H. 2001 . Tetrahedron Lett. , 42 : 6053 – 6055 .
- Kwan , J. and Kim , M.J. 2002 . J. Org. Chem. , 67 : 6845 – 6847 .
- Greene , T.W. ; Wuts , P.G.M. Protective Groups in Organic Synthesis ; Wiley : New York , 1999 .
- Hanson , J.R. Protective Groups in Organic Synthesis ; Blackwell : Malden, MA , 1999 .
- Chakraborti , A.K. ; Nayak , M.K. ; Sharma , L. J. Org. Chem . 2002 , 67 , 1776 1780 ; Chakraborti, A.K.; Nayak, M.K.; Sharma, L. J. Org. Chem. 2002, 67, 2541–2547; Chakraborti, A.K.; Nayak, M.K.; Sharma, L. J. Org. Chem. 1999, 64, 8027–8030 .
- Chakraborti , A.K. , Sharma , L. and Sharma , U. 2001 . Tetrahedron , 57 : 9343 – 9346 .
- Steglich , W. and Hofle , G. 1969 . Angew. Chem. Int. Ed. Engl. , 8 : 981 – 983 .
- Vedjes , E. and Diver , T.S. 1993 . J. Am. Chem. Soc. , 115 : 3358 – 3359 .
- Ahmed , S. and Iqbal , J. 1986 . Tetrahedron Lett. , 27 : 3791
- Chandrasekhar , S. , Ramachander , T. and Takhi , M. 1998 . Tetrahedron Lett. , 39 : 3263
- Chakraborti , A.K. and Gulhane , R. 2003 . Tetrahedron Lett. , 44 : 6749 – 6753 .
- Ishihara , K. , Kubota , M. , Kurihara , H. and Yamamoto , H. 1996 . J. Org. Chem. , 61 : 4560 – 4567 .
- Procopiou , P.A. , Baugh , S.P. , Flack , S.S. and Inglis , G.G.A. 1998 . J. Org. Chem. , 63 : 2342 – 2347 .
- Orita , A. , Tanahashi , C. , Kakuda , A. and Otera , J. 2001 . J. Org. Chem. , 66 : 8926 – 8934 .
- Chauhan , K.K. , Frost , C.G. , Love , I. and Waite , D. 1999 . Synlett , 11 : 1743 – 1744 .
- Dalpozzo , R. , De Nino , A. , Maiuolo , L. , Procopio , A. , Nardi , M. , Bartoli , G. and Romeo , R. 2003 . Tetrahedron Lett. , 44 : 5621 – 5624 .
- Nakae , Y. , Kusaki , I. and Sato , T. 2001 . Synlett , 10 : 1584 – 1586 .
- Chakraborti , A.K. , Sharma , L. and Gulhane , R. 2003 . Tetrahedron , 59 : 7661 – 7668 .
- Bartoli , G. , Bosco , M. , Dalpozzo , R. , Marcantony , E. and Massaccesi , M. 2003 . Synlett , 1 : 39 – 42 .
- Chakraborti , A.K. , Gulhane , R. and Shivani , R. 2003 . Synlett , 12 : 1805 – 1808 .
- Shivani , R. , Gulhane , R. and Chakraborti , A. 2007 . J. Mol. Catal. A Chem. , 264 : 208 – 213 .
- Jeyakumar , K. and Chand , D.K. 2006 . J. Mol. Catal. A Chem. , 255 : 275 – 282 .
- Forsyth , S.A. , MacFarlane , D.R. , Thomson , R.J. and Von Itzestein , M. 2002 . Chem. Commun. , 7 : 714 – 715 .
- Kumareswaran , R. , Pachmuthu , K. and Vankar , Y.D. 2000 . Synlett , 11 : 1652 – 1654 .
- Ballini , R. , Bosica , G. , Carloni , S. and Ciarralli , L. 1998 . Tetrahedron Lett. , 39 : 6049 – 6052 .
- Li , A-X. , Li , T-S. and Ding , T-H. 1997 . Chem. Commun. , 15 : 1389 – 1390 .
- Chakraborti , A.K. and Gulhane , R. 2003 . Tetrahedron Lett. , 44 : 3521 – 3525 .
- Chakraborti , A. K. and Gulhane , R. 2003 . Chem. Commun. , 15 : 1896 – 1897 .
- Qiu , R. , Zhu , Y. , Xu , X. , Li , Y. , Shao , L. , Ren , X. , Cai , X. , An , D. and Yin , S. 2009 . Catal. Commun. , 14 : 1889 – 1892 .
- Xi , Z. , Hao , W. , Wang , P. and Cai , M. 2009 . Molecules , 14 : 3528 – 3537 .
- Joseph , J.K. , Jain , S.L. and Sain , B. 2007 . J. Mol. Cat. A Chem. , 267 : 108 – 111 .
- Sadaphal , S. , Sonar , S. , Ware , M. and Shingare , M. 2008 . Green Chem. Lett. Rev. , 1 : 191 – 196 .
- Sadaphal , S. , Sonar , S. , Kategaonkar , A. and Shingare , M. 2009 . Bull. Korean Chem. Soc. , 30 ( 5 ) : 1054 – 1056 .
- Sonar , S. , Sadaphal , S. , Pawar , S. , Shingate , B. and Shingare , M. 2009 . Chin. Chem. Lett. , 20 : 557 – 561 .
- Hajipour , A. , Nasreesfahani , Z. and Ruoho , A. 2008 . Org. Prep. Proced. Int. , 40 : 385 – 391 .
- Wang , W. , Cheng , W. , Shao , L. and Yang , J. 2008 . Catal. Lett. , 121 : 77 – 80 .
- Bhattacharya , A.K. , Diallo , M.A. and Ganesh , K.N. 2008 . Synth. Commun. , 38 : 1518 – 1526 .
- Zhang , L. , Luo , Y. , Fan , R. and Wu , J. 2007 . Green Chem. , 9 : 1022 – 1025 .
![Figure 1. Morpholinium bisulfate ([morH][HSO4]).](/cms/asset/a7112a26-ae7b-4aba-97da-56b9dae3b0e4/tgcl_a_504751_o_f0001g.gif)