Abstract
Bernthsen reaction has been carried out under microwave irradiation in the presence of p-TSA (10 mol%) as catalyst in a solventless reaction to provide 9-substituted acridines. In the present report, time economy and better yields as compared to conventional Bernthsen reaction are described.
Introduction
Acridines represent an important class of nitrogen heterocycles Citation1 Citation2 having several significant properties such as pigment and dye properties Citation3–5, photochemical/physical properties Citation6–8, electrochemical properties Citation9, potent anti-malarial activity Citation10, anticancer activity Citation11–16,Footnote1 antifungal activity Citation17–20, etc. Natural and synthetic acridines and their derivatives are effective DNA and RNA-binding compounds Citation21–24 owing to their intercalation abilities as well as being a lipophilic carrier molecule. It is the acridine chromophore that renders to the molecules a planar structure allowing them to bind DNA by stacking between base pairs. Recently, structure–activity relationship of acridine analogs as haspin and DYRK2 kinase inhibitors has been studied Citation25.
In one of our ongoing synthetic programs on bioactive molecules and organic synthesis involving microwave energy, which provides greener reaction condition coupled with increased yield and time economy, we came across Bernthsen reaction. Owing to the bioactivity of acridines, we became interested therein. Use of microwave energy for the enhancement of organic reactions, i.e. microwave organic reactions enhancement (MORE) is well known Citation26–28. That is why, we decided to explore the same in our ongoing program on bioactive molecules.
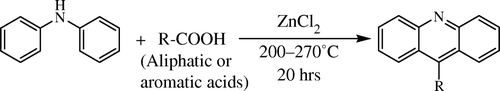
Acridine family members can be prepared by classical Bernthsen reaction by coupling an carboxylic acid (aromatic or aliphatic) and diphenylamine (DPA) in the presence of zinc chloride () at a temperature of 200–210°C for about 20 hours Citation29 Citation30. The conditions for this reaction tend to be quite vigorous coupled with very low yield. In addition, more than stoichiometric amounts of ZnCl2 is required [1:5:1 (DPA:ZnCl2:carboxylic acid)]. In 1962, Popp reported a modified Bernthsen reaction procedure by replacing the Lewis acid ZnCl2 with polyphosphoric acid Citation31, which was more convenient in regard to reaction times despite the poorer yields. Toma et al. Citation32 and Seijas et al. Citation33 have reported an improved synthesis of acridine derivatives assisted by microwave and catalyzed by ZnCl2. But, as compared to synthetic methodologies for other classes of compounds, this reaction has not been explored adequately as evidenced by reports of only two catalysts (ZnCl2 and polyphosphoric acid) being employed.
But none of these procedures were satisfactory in terms of yield and time economy. In this regard, hence, there is still need for looking at this reaction in order to have improved reaction conditions/superior catalysts so that better yields could be achieved. At the same time, we should not forget the cost, environment, etc. Accordingly, our main aim of this present investigation is to find alternative conditions/catalysts for Bernthsen reaction with the following objectives: (a) to search for milder reaction condition, (b) to reduce the reaction time, (c) to increase the yield, (d) to promote economical and environmentally friendly experimental procedures (green chemistry) by performing the reaction under microwave and solventless condition, and (e) to use cost-effective, easily available, mild, water tolerant, and economical catalysts alternative to ZnCl2 and polyphosphoric acid.
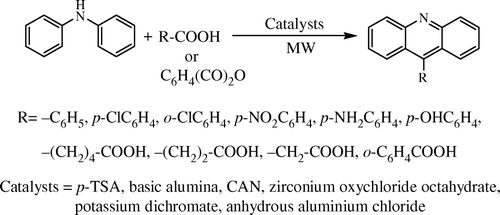
In an effort to amend the situation above, in this communication, we report a simple and general solventless reaction for the synthesis of 9-substituted acridines, by a modification of classical Bernthsen reaction (), using p-TSA (p-toluenesulphonic acid) as the catalyst under microwave irradiation. This paves the way for an environmentally benign condition without compromising viability and speed. Our method is equally applicable to both aliphatic and aromatic carboxylic acids, thereby providing more generality and flexibility.
An inspection of the literatures revealed that in Bernthsen reaction using ZnCl2 and polyphosphoric acid, the stoichiometry used for DPA and carboxylic acid was not 1:1. The acid was taken always in excess. The catalyst used was also in stoichiometric amount or higher. In our green chemistry approach, we searched for the conditions where the substrates would be used in stoichiometric ratios and the catalyst used would be in minimum amount, e.g. (10 mol% or lower than that).
Results and discussion
Other than p-TSA, we studied the effects of several compounds as catalyst, e.g. basic Al2O3, cerric ammonium nitrate (CAN), ZrOCl2 8H2O, K2Cr2O7, anhydrous AlCl3 in Bernthsen reaction (reaction of DPA and benzoic acid was chosen as the model reaction) under thermal as well as MWI in the absence of solvent. However, the best results in terms of yields and reaction time were obtained with p-TSA () under MWI. CAN and basic alumina also afforded the products but yields were poorer. In order to demonstrate the scope of this reaction, differently substituted benzoic acids, aliphatic mono/dicarboxylic acids were taken up for the systematic study which is depicted in . However, in the conditions we explored, it was found that in the ratio of 1:1 (DPA and carboxylic acid), only entries 1 and 11 () worked well; in all other cases the yields were not satisfactory. Too many products were observed in TLC and also we faced difficulty in separation by column chromatography. The best results were obtained when we used the acids in twofolds with respect to DPA. To our delight, we were successful in reducing the amount of catalyst from stoichiometric ratios to 10 mol%, a significant outcome in comparison to the classical methods. Aromatic anhydride also responded and showed reactivity under this condition (entry 11). p-Nitro (entry 4) and p-Amino benzoic acids (entry 5) also afforded acridine under the reaction condition. Overheating and increasing the reaction time did not increase the reaction yield; rather some polymeric products were obtained.
Table 1. Screening of catalysts for the synthesis of 9-(phenyl)acridine via Scheme 2.
Table 2. p-TSA catalyzed synthesis of acridine derivatives via Scheme 2.
In conclusion, nontraditional MORE of Bernthsen acridine synthesis is achieved in a simple, clean, fast, and solventless reaction catalyzed by p-TSA. Good time economy and better yields as compared to conventional Bernthsen reaction was observed. The reaction is applicable to aromatic as well as aliphatic acids (mono- and di-).
Considering the environmental issues that require the substitution of toxic catalysts by more friendly catalysts, our methodology is consistent with green chemistry philosophy Citation34. Moreover, p-TSA is easily available and can be easily removed from the reaction mixture being water-soluble.
Experimental
Melting points were determined on a Buchi 504 apparatus and are uncorrected. IR spectra were recorded in KBr pallets on a Nicolet (Impact 410) FT-IR spectrophotometer. 1H NMR and 13C NMR spectra were recorded on a Varian Mercury Plus 400 MHz NMR spectrophotometer using tetramethylsilane (TMS) as internal standard. Coupling constants are expressed in hertz. Microwave synthesis system used was model CAT-2R from CatalystTM systems. The progress of the reaction was monitored by thin layer chromatography (TLC) run on silica gel G (Merck). All the chemicals were used as received. Elemental analysis was done in a Perkin Elmer CHN analyzer (2400 series II).
General procedure for the synthesis of 9-(phenyl)acridine
A mixture of N,N-DPA (169 mg, 1 mmol), benzoic acid (122 mg, 1 mmol), and pure p-TSA (19 mg, 10 mol%) was irradiated in a microwave oven (450 W) for 5 mins. The reaction was monitored by TLC. The crude product was extracted with chloroform (20 ml×2) and washed with 10% NaOH followed by distilled water. The organic layer was dried over Na2SO4, evaporated and the residue was subjected to column chromatography to give 9-phenylacridine (244.80 mg, 80% yield). The pure products were characterized by IR, 1H-NMR, 13C-NMR spectroscopy and mass spectrometry, elemental analysis and mp.9-(phenyl)acridine (, entry 1)
IR (KBr) (v max/cm−1) 1652.64 (C = N); 1H NMR (400 MHz, CDCl3) δH 7.42–7.46 (m, 4H, Ar-H), 7.59–7.61 (m, 3H, Ar-H), 7.71 (d, 2H, J=4.4 Hz, Ar-H), 7.76–7.80 (m, 2H, Ar-H), 8.28 (d, 2H, J=4.4 Hz, Ar-H); 13C NMR (100 MHz, CDCl3) δc 125.2, 125.6, 126.9, 128.4, 128.5, 129.7, 130.0, 130.5, 135.9, 147.1, 148.7 ppm. MS, m/z 255 (M+). Elemental analysis for C19H13N: Calculated, C, 89.38; H, 5.13; N, 5.49. Found: C, 88.47; H, 5.09; N, 6.01. mp. 183–185°C (lit.16 mp. 184–185°C).
Similarly, the other products were also synthesized () and characterized (supplementary information).
Supplementary information
Data for all the compounds are given as supplementary information.Supplementary information
Acknowledgement
AJT thanks Department of Science and Technology (DST), Govt. of India, New Delhi, for financial support to the project (SR/FTP/CS-58/2005) and UGC, New Delhi, for a Rajiv Gandhi National Fellowship to SD.
Notes
1. For chemotherapeutic effects of acridine derivatives, see (Citation13).
References
- Albert , A. The Acridines ; Edward Arnold : London , 1966
- Acheson , R.M. Acridines. The Chemistry of Heterocyclic Compounds ; Interscience Publishers : New York 1956
- Katritzky , A.R. Handbook of Heterocyclic Chemistry ; Pergamon Press : Oxford 1985
- Shanmugasundaram , P. ; Prabahar , K.J. ; Ramakrishnan , V.T. J. Heterocycl. Chem. 1993 , 30 , 1003 1007 .
- Islam , A. ; Murugan , P. ; Hwang , K.C. ; Cheng , C.-H. Synth. Metals 2003 , 139 , 347 353 .
- Mohan , H. ; Srividya , N. ; Ramamurthy , P. ; Mittal , J.P. J. Phys. Chem. 1997 , 101 , 2931 2935 .
- Srividya , N. ; Ramamurthy , P. ; Ramakrishnan , V.T. Spectrochim. Acta Part A 1998 , 54 , 245 253 .
- Mohan , H. ; Mittal , J.P. ; Srividya , N. ; Ramamurthy , P. J. Phys. Chem. 1998 , 102 , 4444 4449 .
- Srividya , N. ; Ramamurthy , P. ; Shanmugasundaram , P. ; Ramakrishnan , V.T. J. Org. Chem. 1996 , 61 , 5083 5089 .
- Dominguez , J.N. ; Lopez , S. ; Charris , J. ; Iarruso , L. ; Lobo , G. ; Semenov , A. ; Olson , J.E. ; Rosenthal , P.J. J. Med. Chem. 1997 , 40 , 2726 2732 .
- Demeunynck , M. ; Charmantray , F. ; Martelli , A. Curr. Pharm. Des. 2001 , 7 , 1703 1724 .
- Santelli-Rouvier , C. ; Barret , J.M. ; Farrell , C.M. ; Sharples , D. ; Hill , B.T. ; Barbe , J. Eur. J. Med. Chem. 2004 , 39 , 1029 1038 .
- Denny , W.A. Med. Chem. Rev. Online 2004 , 1 , 257 266 .
- Denny , W.A. ; Baguley , B.C. Curr. Top. Med. Chem. 2003 , 3 , 339 353 .
- Moloney , G.P. ; Kelly , D.P. ; Mack , P. Molecules 2001 , 6 , 230 243 .
- Chilin , A. ; Marzaro , G. ; Marzano , C. ; Dalla , V.L. ; Ferlin , M.G. ; Pastorini , G. ; Guiotto , A. Bioorg. Med. Chem. 2009 , 17 , 523 529 .
- Wu , B.J. ; Thompson , S.T. Appl. Environ. Microbial. 1984 , 48 , 743 746 .
- Chaube , K.R. ; Ragini , K. ; Dixit , S.N. ; Tripathi , S.C. Pesticides 1984 , 18 , 28 29 .
- Volyanskii , Y.L. ; Meínik , M.V. ; Gutsulyak , B.M. Khim.-Farm. Zh. 1979 , 13 , 36 40 .
- Monte , W. ; Stamm , J. Tetrahedron Lett. 1993 , 34 , 7161 7162 .
- Sivaraman , J. ; Subramanian , K. ; Ganesan , S. ; Ramakrishnan , V.T. J. Biomol. Struct. Dyn. 1995 , 13 , 119 134 .
- Hopcroft , N.H. ; Brogden , A.L. ; Searcey , M. ; Cardin , C. Nucl. Acids Res. 2006 , 4 , 6663 6672 .
- Lerman , L.S. J. Mol. Biol. 1961 , 3 , 18 30 .
- Adams , A. ; Guss , J.M. ; Collyer , C.A. ; Denny , W.A. ; Prakash , A.S. ; Wakelin , L.P.G. Mol. Pharmacol. 2000 , 58 , 649 658 .
- Cuny , G.D. ; Robin , M. ; Ulyanova , N.P. ; Patnaik , D. ; Pique , V. ; Casano , G. ; Liu , J.-F. ; Lin , X. ; Xian , J. ; Glicksman , M.A. ; Stein , R.L. ; Higgins , J.M.G. Bioorg. Med. Chem. Lett. 2010 , 20 ( 12 ), 3491 3494 .
- Polshettiwar , V. ; Varma , R. Acc. Chem. Res. 2008 , 41 , 629 639 .
- Caddick , S. Tetrahedron 1995 , 51 , 10403 10432 .
- Loupy , A. ; Petit , A. ; Hamelin , J. ; Texier-Boullet , J. ; Jacquault , F. ; Methe , D. Synthesis 1998 , 9 , 1213 1234 .
- Bernthsen , A. Justus Liebiğgs Ann. Chem. 1984 , 224 , 1 56 .
- Buu-Hoi , N.P. ; Royer , R. ; Hubert-Habart , M. J. Chem. Soc. 1955 , 1082 1084 .
- Popp , F.D. J. Org. Chem. 1962 , 22 , 2658 2659 .
- Veverková , E. ; Nosková , M. ; Toma , S. Synth. Commun. 2002 , 32 , 729 733 .
- Seijas , J.A. ; Vázquez-Tato , M.P. ; Martínez , M.M. ; Rodríguez-Parga , J. Green Chem. 2002 , 4 , 390 391 .
- Anastas , P.T. ; Warner , J.C. Green Chemistry: Theory and Practice ; Oxford University Press : Oxford , 2000
Supplementary information
9-(4-chlorophenyl)acridine (, entry 2)
IR (KBr) (v max/cm−1) 1650.41 (C = N); 1H NMR (400 MHz, CDCl3) δH 7.13 (d, 2H, J=3.25 Hz, Ar-H), 7.17 (t, 2H, J=3.05 Hz, Ar-H), 7.26 (d, 2H, J=3.28 Hz, Ar-H), 7.40–8.06 (m, 6H, Ar-H); 13C NMR (100 MHz, CDCl3) δc 125.5, 126.2, 126.5, 127.4, 128.5, 129.6, 130.0, 133.4, 135.2, 142.6, 168.4 ppm. MS, m/z 289 (M+). Elemental analysis for C19H12ClN: Calculated, C, 78.76; H, 4.17; N, 4.83. Found: C, 79.07; H, 4.25; N, 4.76. mp. 245–248°C.
9-(2-chlorophenyl)acridine (, entry 3)
IR (KBr) (v max/cm−1) 1651.01 (C = N); 1H NMR (400 MHz, CDCl3) δH 7.33–7.42 (m, 4H, Ar-H), 7.59–7.63 (m, 4H, Ar-H), 7.77–8.17 (m, 4H, Ar-H); 13C NMR (100 MHz, CDCl3) δc 125.3, 126.3, 126.5, 127.2, 128.7, 129.8, 130.3, 132.2, 135.1, 144.1, 160.3 ppm. MS, m/z 289 (M+). Elemental analysis for C19H12ClN: Calculated, C, 78.76; H, 4.17; N, 4.83. Found: C, 79.07; H, 4.27; N, 4.84. mp. 269–271°C.
9-(4-nitrophenyl)acridine (, entry 4)
IR (KBr) (v max/cm−1) 1646.91 (C = N), 1523 (–NO2); 1H NMR (400 MHz, CDCl3) δH 7.46 (t, 2H, J=3.09 Hz, Ar-H), 7.61–7.64 (m, 6H, Ar-H), 8.16 (d, 2H, J=3.17 Hz, Ar-H), 8.21 (d, 2H, J=3.17 Hz, Ar-H); 13C NMR (100 MHz, CDCl3) δc 124.7, 125.1, 126.4, 128.1, 128.5, 129.7, 130.1, 135.8, 139.4, 146.9, 162.6 ppm. MS, m/z 300 (M+). Elemental analysis for C19H12N2O2: Calculated, C, 75.99; H, 4.03; N, 9.33. Found: C, 74.98; H, 4.01; N, 8.89. mp. 258–260°C.
9-(4-aminophenyl)acridine (, entry 5)
IR (KBr) (v max/cm−1) 3334 (–NH2), 1650.46 (C = N); 1H NMR (400 MHz, CDCl3) δH 4.07 (br, s, 2H, NH2), 7.26 (t, 2H, J=3.18 Hz, Ar-H), 7.61–7.64 (m, 6H, Ar-H), 8.14 (d, 2H, J=3.17 Hz, Ar-H), 8.23 (d, 2H, J=3.17 Hz, Ar-H); 13C NMR (100 MHz, CDCl3) δc 124.9, 125.4, 126.7, 128.2, 128.6, 129.9, 130.2, 135.8, 137.7, 147.1, 148.5 ppm. MS, m/z 270 (M+). Elemental analysis for C19H14N2: Calculated, C, 84.42; H, 5.22; N, 10.36. Found: C, 84.47; H, 5.31; N, 9.98. mp 270–272°C.
9-(4-hydroxyphenyl)acridine (, entry 6)
IR (KBr) (v max/cm−1) 3632 (–OH), 1650.46 (C = N); 1H NMR (400 MHz, CDCl3) δH 7.05–7.35 (m, 6H, Ar-H), 7.56–7.97 (m, 6H, Ar-H), 8.68 (br, s, 1H, –OH); 13C NMR (100 MHz, CDCl3) δc 124.9, 125.4, 126.7, 128.2, 128.6, 129.9, 130.2, 135.8, 137.7, 147.1, 148.5 ppm. MS, m/z 271 (M+). Elemental analysis for C19H13NO: Calculated, C, 84.11; H, 4.83; N, 5.16. Found: C, 84.10; H, 4.85; N, 5.07. mp >300°C.
2-(acridin-9-yl)benzoic acid (, entries 7 & 11)
IR (KBr) (v max/cm−1) 3456 (OH), 1737.41 (C = O), 1652.64 (C = N); 1H NMR (400 MHz, CDCl3) δH 7.55 (t, 2H, J=3.01 Hz, ArH), 7.70 (t, 2H, J=3.01 Hz, ArH), 8.14–8.28 (m, 4H, ArH); 13C NMR (100 MHz, CDCl3) δc 119.3, 125.9, 126.8, 127.4, 128.1, 128.3, 128.7, 132.8, 138.1, 141.3, 147.9, 191.9 ppm. MS, m/z 299 (M+). Elemental analysis for C20H13NO2: Calculated, C, 80.25; H, 4.38; N, 4.68. Found: C, 81.01; H, 4.54; N, 4.31. mp. 265–266°C.
2-(acridin-9-yl)acetic acid (, entry 8)
IR (KBr) (v max/cm−1) 3446 (OH), 1731.83 (C = O), 1637.76 (C = N); 1H NMR (400 MHz, CDCl3) δH 2.85 (s, 2H, CH2), 7.51 (t, 2H, J=2.98 Hz, ArH), 7.67 (t, 2H, J=2.99 Hz, ArH), 8.10–8.23 (m, 4H, ArH); 13C NMR (100 MHz, CDCl3) δc 54.0, 124.3, 125.5, 127.5, 128.5, 129.3, 135.4, 147.5, 200.3 ppm. MS, m/z 237 (M+). Elemental analysis for C15H11NO2: Calculated, C, 75.94; H, 4.67; N, 5.90. Found: C, 75.86; H, 4.78; N, 5.79. mp. Decomp.
3-(acridin-9-yl)propanoic acid (, entry 9)
IR (KBr) (v max/cm−1) 3440 (OH), 1732.96 (C = O), 1638.91 (C = N); 1H NMR (400 MHz, CDCl3) δH 2.50 (t, 2H, J=3.01 Hz, CH2), 2.71 (t, 2H, J= 3.02 Hz, CH2COOH), 7.54 (t, 2H, J= 2.93 Hz, ArH), 7.66 (t, 2H, J=2.93 Hz, ArH), 8.00–8.17 (m, 4H, ArH); 13C NMR (100 MHz, CDCl3) δc 27.0, 35.6, 124.9, 125.8, 127.6, 128.8, 129.3, 135.3, 147.1, 200.7 ppm. MS, m/z 251 (M+). Elemental analysis for C16H13NO2: Calculated, C, 76.48; H, 5.21; N, 5.57. Found: C, 77.13; H, 5.23; N, 5.61. mp 305–3078°C.
5-(acridin-9-yl)pentanoic acid (, entry 10)
IR (KBr) (v max/cm−1) 3442 (OH), 1731.61 (C = O), 1637.13 (C = N); 1H NMR (400 MHz, CDCl3) δH 1.24–1.20 (m, 4H, 2CH2), 1.98 (t, 2H, J=2.99 Hz, CH2-COOH), 2.11 (t, 2H, J= 2.97 Hz, ArCH2), 7.58 (t, 2H, J=2.97 Hz, ArH), 7.69 (t, 2H, J=2.96 Hz, ArH), 8.05–8.07 (m, 4H, ArH); 13C NMR (100 MHz, CDCl3) δc 22.8, 27.0, 35.6, 54.0, 124.9, 125.8, 127.4, 128.6, 129.5, 135.3, 147.1, 200.3 ppm. MS, m/z 279 (M+). Elemental analysis for C19H21NO2: Calculated, C, 77.40; H, 6.13; N, 5.0. Found: C, 77.27; H, 5.98; N, 4.91. mp. 265–69°C.