Abstract
An expedient method of silica supported heteropolyacid (HPA) catalyzed liquid phase dehydration of aldoxime to nitrile and secondary alcohols to alkenes were developed. Supported HPA was prepared by wet impregnation method and characterized by various analytical techniques such as X-ray diffraction, FT-IR, and NH3-TPD analysis. HPW-SiO2 was found to be the most efficient catalyst amongst the prepared HPA and was found to be reusable for dehydration reaction under mild operating conditions giving high yields of the desired products.
Introduction
Nitriles are useful precursors for synthesis of amines, amides, ketones, carboxylic acids, and esters. They also act as key intermediates for several pharmaceuticals, agrochemicals, dyes, and electro-optical materials Citation1–6. Nitriles are also used as precursors of tetrazole analogs, which are used as antipicornavirus drugs Citation7, triazo [1,5-c] pyrimidines with potential antiasthama activity Citation8, and commercially important angiotensin 1 receptor ligands, losartan, and valsartan Citation9. The traditional method for the synthesis of aromatic nitriles from aryl bromides/iodides requires a stoichiometric amount of Cu (I) cyanide at elevated temperatures Citation10 Citation11. Recently, Pd-based catalytic methods have acquired attention due to their functional group tolerance and high catalytic activity Citation12–14. However, the methodology suffers from various problems such as toxicity and tedious work up procedures. Alternatively, nitriles are also synthesized by dehydration of aldoximes and this transformation has been widely studied. Various catalytic systems including CoCl2 Citation15, KF/Al2O3 Citation16, W–Sn hydroxide Citation17, ZnO/CH3COCl Citation18, metal triflates Citation19 Citation20, clay Citation21, and zeolites Citation22, are reported for dehydration of aldoximes to nitriles. Some non-conventional techniques like use of microwaves Citation23 Citation24 have also been reported. Inspite of the recent developments, existing methodologies suffer from one or more drawbacks such as tedious work up procedures, long-reaction time, use of additive (base), and lower yield of the desired nitriles.
To affect such transformation supported heteropolyacid (HPA) are the best candidates as they exhibit interesting properties such as high acidity nearing super acidity region, easy work up procedures, and recyclability. Supported HPA has proven to be the efficient catalysts for several organic transformations such as C-alkylation Citation25, esterification Citation26, Ritter reaction Citation27, Bigenelli reaction Citation28, and Dakin–West reaction Citation29. Our group has also explored the catalytic activity of HPA for various other transformations Citation30 Citation31. In continuation of our work on HPA, we herein report an expedient method of silica supported HPA catalyzed liquid phase dehydration of aldoximes to nitriles under mild reaction conditions. The catalytic protocol was further extended for dehydration of secondary alcohols to alkenes.
Results and discussions
Study of physio-chemical properties
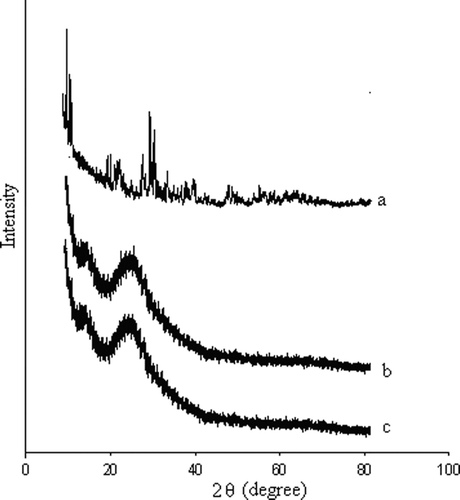
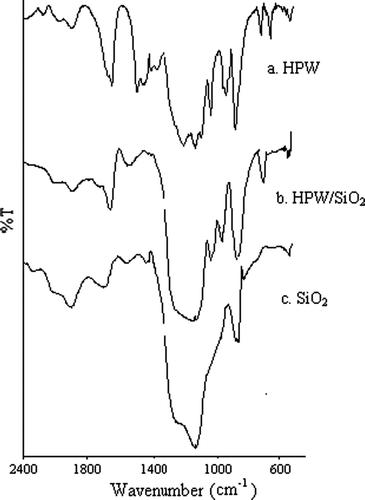
The prepared catalysts were well-characterized by analytical tools such as X-ray diffraction (XRD) () FT-IR Citation32, NH3-TPD Citation33 (Table 1a), and BET surface area measurements. The results corroborate that 12-tungstophosphoric acid (HPW) is present on the SiO2 with an undegraded structure. All the related data have been well-discussed in the supporting information.
Catalytic studies
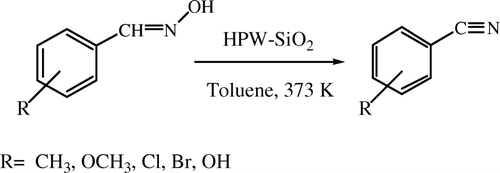
Initially, dehydration of benzaldehyde oxime to benzonitrile was chosen as the model reaction () and effect of various reaction parameters such as influence of different HPAs, influence of solvents, influence of HPA loading, influence of catalyst concentration, and effect of temperature were studied to give the optimum results.
Influence of different supported heteropolyacids (HPAs)
To start with the reaction system, various HPA-based catalysts were screened. As shown in , SiO2 showed very poor yield of 10% (entry 1) and hence it can be inferred that it did not play any significant role in the reaction mechanism other than support to HPA. Bulk HPW showed better results than 12-molybdophosphoric acid (HPM) giving moderate yields of 51% and 39% (entries 2 and 3), respectively. Similar trend was observed when both these acids were supported on SiO2, HPW-SiO2 gave 85% of benzonitrile, whereas HPM-SiO2 gave 72% yield (entries 4 and 5). However, when reaction was carried out without catalyst no product formation was observed. Thus, HPW-SiO2 was used as a catalyst for further studies.
Table 1. Influence of heteropolyacids on dehydration of aldoximes.a
Influence of solvent
It was observed that a solvent plays a crucial role in the activity and performance of the catalyst thus, choice of a proper solvent was necessary. Influence of various solvents on the reaction system was investigated (). It was observed that polar protic solvents such as ethanol and water (entries 1 and 2) were found to be ineffective under the given conditions. With a polar protic solvent, the substrate did not have too much chance to come in contact with the catalyst, therefore the conversion was found to be low. Similar results were earlier reported by Ghiaci et al. Citation34 Polar aprotic solvent such as acetonitrile and DMF gave moderate yields of 65% (entry 3) and 62% (entry 4), respectively. However, non-polar solvent such as toluene was found to be the best solvent providing 85% yield (entry 5) and was further used as a reaction medium.
Table 2. Influence of solvent on dehydration of aldoximes.a
Influence of catalyst concentration
Variation of HPW loading on silica between 0 and 30 wt% had different effects on the activity for dehydration of benzaldehyde oxime. The activity of the catalysts with different HPW loadings is compared in . The results obtained showed that as the loading of HPW was increased from 5 to 20% (entries 1–4), yield of benzonitrile was also increased from 38 to 85%. On further increasing the loading to 25% the yield remained constant (entry 5). But when the loading of HPW was increased to 30% the yield of nitrile decreased to 82% (entry 6). It was assumed that by increasing the loading after an optimum-level HPW might start adsorbing on the already adsorbed HPW molecules thus decreasing the active catalytic sites for reaction. Thus 20% loading of HPW was chosen as the optimum loading on SiO2 support. Similar trend can also be traced with the amount of acidity measured on these prepared catalysts.
Table 3. Influence of heteropolyacid loading on SiO2 for dehydration of aldoximes.a
Next, the amount of catalyst loading on the yield of nitrile was studied and it was observed that as the amount of catalyst loading was increased from 10 to 20 wt% (, entries 1–3) the yield of desired nitrile steadily increased. Whereas, on further increasing the catalyst loading till 30 wt% (entries 4 and 5) no appreciable increase in yield was observed and hence 20 wt% was chosen to be the best catalyst concentration and was used further for dehydration reactions.
Table 4. Influence of catalyst concentration on dehydration of aldoximes.a
Influence of temperature
The effect of temperature on dehydration of benzaldehyde oxime was investigated. The reaction was studied at four different temperatures viz., 333 K, 353 K, 373 K, and 393 K (). The yield of benzonitrile was found to increase with the increase in the temperature and highest yield was obtained at 373 K (, entries 1–3). With further increase in the reaction temperature to 393 K (entry 4) the yield of benzonitrile remained constant and hence 373 K was used as an optimal temperature.
Table 5. Effect of temperature on dehydration of aldoximes to nitriles.a
Catalytic dehydration of aldoximes to nitriles
The optimized reaction conditions were then applied to diverse aldoximes in order to study the generality of the protocol. Initially, synthesis of benzonitrile from benzaldehyde oxime was chosen as a test reaction and it was found that it gave good yield of 85% (, entry 1). The protocol was then applied for synthesis of substituted nitriles and it was found that electron donating groups such as -CH3 and -OCH3 gave excellent yields of 88 and 92% of their respective nitriles (, entries 4 and 5). The electron withdrawing substituents such as Cl- and Br- on aromatic aldoximes were tested and good yields of 4-Cl-benzonitrile (80%) and 4-Br-benzonitrile (78%) were obtained (entries 3 and 4). When 4-hydroxy-3-methoxy benzaldehyde oxime was dehydrated using the present reaction conditions 82% of 3-hydroxy-2-methoxy benzonitrile (entry 5) was formed. It was observed that cinnamaldoxime also reacted smoothly providing good yields upto 81% of cinnamonirile (, entry 7). However, aliphatic aldoximes such as vaeraldehyde oxime (entry 8) and crotonaldehyde oxime (entry 9) gave moderate yields of their respective nitriles.
Table 6. HPW-SiO2 catalyzed conversion of aldoximes of nitriles.a
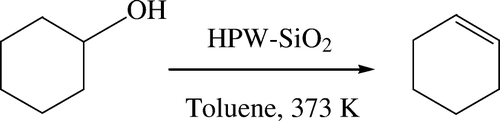
Catalytic dehydration of secondary alcohols to alkenes
Very few methodologies are reported for liquid phase dehydration of alcohols to alkene. The direct dehydration of an alcohol to alkene is a major issue since it requires high temperature and acidic conditions leading to various side products Citation35. Thus, encouraged by the results obtained for dehydration of aldoximes to nitriles, dehydration of secondary alcohols to alkenes () was also tested under the same set of reaction conditions ().The methodology was first tested for dehydration of alicyclic alcohols such as cyclohexanol and cycloheptanol and was found to give excellent yields ranging from 92 to 95% (, entries 1 and 2). Benzylic alcohols such as 1-phenethyl alcohol and 4-methoxy-1-phenethyl alcohol were found to be sluggish providing lower yields of alkenes due to formation of their cyclodimers (entries 3 and 4). Whereas, sterically hindered alcohols such as 1,2,3,4-tetrahydronaphthol and 1-indanol (entries 5 and 6) were found to react smoothly under the present conditions providing good yields of the corresponding alkenes.
Table 7. HPW-SiO2 catalyzed conversion of secondary alcohols to alkenes.a
Catalyst reusability
An important criterion for heterogeneous catalysis is the reusability. Hence series of reaction cycles were studied in order to investigate the stability of the catalytic system.
The test for leaching of HPA was performed, wherein the catalyst was filtered after partial conversion and the reaction was further allowed to react for the optimized time. It was observed that the reaction did not proceed further after the removal of catalyst, which implies that no leaching of HPA was observed.
To study the catalyst reusability, dehydration of benzaldehyde oxime was carried out on HPW-SiO2 several times. After the reaction was completed, the catalyst was filtered under vacuum washed with toluene, acetone, dried at 373 K for 1 h, and was used again for the next cycle. The results of four consecutive cycles are shown in (entries 1–4). From these results it can be inferred that there was no appreciable loss in catalytic activity and the catalytic system was stable upto four cycles.
Table 8. Reusability of HPW-SiO2 for dehydration reactions.
Experimental
Materials and methods
All chemicals were procured from firms of repute. Various aldehydes were purchased from Sigma-Aldrich. Toluene was purchased from S.D. Fine Chem. India Ltd. All the chemicals were used as received without any further purification. Powder XRD of the catalyst was recorded with a diffractometer (Philips 1050) using graphite monochromatized Cu-Kα radiation over a 2θ range of 10–90°. NH3-TPD technique was used to assess the acid strength of the catalyst using a conventional flow reactor on Micrometrics microbalance using 20 mg sample of the catalyst and a commercial 10% NH3 in He gas mixture. BET surface area measurements were carried out by nitrogen adsorption (Micromeritics ASAP 2010) at an adsorption temperature of 77 K. The FTIR spectra were recorded using a Perkin Elmer (Spectra 100) spectrometer by KBr pellet technique. 1H NMR and 13C NMR spectra were recorded on JEOL-300 MHz NMR spectrometer using TMS as an internal standard. GC–MS spectra were recorded on Schimatzu-QP2010.
Preparation of aldoximes
Aldoximes, except 2-hydroxybenzaldoxime, were readily prepared in quantitative yields from corresponding aldehydes. Typically, aldehyde (10 mmol), hydroxylamine hydrochloride (1.4 g, 20 mmol), and sodium acetate trihydrate (2.72 g, 20 mmol) were placed in a mixture of methanol (55 ml) and water (5 ml), and stirred at ambient temperature for about 3 h. Upon the completion of reaction, the solvent was removed under reduced pressure and the resulting residue was dissolved in ethyl acetate (50 ml), and then washed with water. The organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated in vacuum. The oxime, if liquid, was used without further purification and the solid oximes were dried at 50–100°C for 2 h, before use Citation15.
Catalyst preparation
H3PW12O40.nH2O (HPW) supported on silica was prepared using a well-reported impregnation technique Citation36. Impregnating solution was prepared by dissolving H3PW12O40.nH2O in the solvent mixture of demineralized water and absolute ethanol (1:1 volume ratio) at room temperature (solution concentration employed was 120 g of Tungsten/l). A 20% HPW-SiO2 was prepared with appropriate loading of the above HPW solution on the silica support. The support was added to the solution to form a suspension which was stirred vigorously for 72 h. The solid catalyst was separated by centrifugation, dried at room temperature for 24 h, and calcined at 200°C for 3.5 h. The solid thus obtained was then ground to fine particles and washed with the solvent in which the reactions were to be carried out (toluene) and further calcined under the same conditions. The catalyst is denoted as HPW-SiO2.
Typical procedure for the synthesis of nitriles/alkenes
The dehydration reaction of aldoximes was carried out in a 25 ml two neck round bottom flask containing the mixture of aldoxime (2 mmol), HPW-SiO2 (20 wt%), and toluene (3 ml). The mixture was magnetically stirred at 100°C for 4.5 h, the progress of the reaction was monitored by TLC analysis. After completion of the reaction, catalyst was filtered and the filtrate was passed through a bed of anhydrous Na2SO4 to remove the water formed in the reaction. The filtrate was further analyzed by GC and GC–MS. All the prepared compounds are known and were confirmed with the authentic samples. Selected products were characterized with the help of 1H and 13C-NMR.
Conclusions
In conclusion, HPW-SiO2 was found to be a heterogeneous and environmentally benign catalyst for dehydration of aldoximes to nitriles. Diverse aldoximes with different substituents were dehydrated to give their corresponding nitriles. The present catalytic protocol was also successfully applied for liquid phase dehydration of secondary alcohols to alkenes. HPW-SiO2 as a catalyst offers several advantages such as reusability, higher yields of the desired products, and high reaction rates thus provides a good alternative for the existing catalytic systems.
Acknowledgements
The financial assistance from University Grant Commission (UGC), Government of India is kindly acknowledged.
References
- Friederich , K. ; Wallenfels , K. In The Chemistry of the Cyano Group Rappaport Z. New York : Wiley-Interscience , 1970 , 717 742 .
- Fatiadi , A.J. In Preparation and Synthetic Applications of Cyano Compounds Patai S. Rappaport Z. Wiley : New York , 1983 , 1345 1390 .
- Miller , J.S. ; Manson , J.L. Acc. Chem. Res . 2001 , 34 , 563 570 .
- Sundermeier , M. ; Zapf , A. ; Beller , M. ; Sans , S. Tetrahed. Lett . 2001 , 42 , 6707 6710 .
- Fustero , S. ; Salavert , E. ; Sanz-Cervera , J.F. ; Piera , J. ; Asensio , A. Chem. Commun . 2003 , 7 , 844 845
- Ozaki , S. Med. Res. Rev . 1996 , 16 , 51 86 .
- Diana , G.D. ; Cutcliffe , D. ; Volkots , D.L. ; Mallamo , J.P. ; Bailey , T.R. ; Vescio , N. ; Oglesby , R.C. ; Nitz , T.J. ; Wetzel , J. ; Giranda , V. ; Pevear , D.C. ; Dutko , F.J. J. Med. Chem . 1993 , 36 , 3240 3250 .
- Medwid , J.B. ; Paul , R. ; Baker , J.S. ; Brockman , J.A. ; Du , M.T. ; Hallett , W.A. ; Hanifin , J.W. ; Hardy , R.A. Jr ; Tarrant , M.E. ; Torley , L.W. ; Wrenn , S. J. Med. Chem . 1990 , 33 , 1230 1241 .
- Khanna , I.K. ; Weier , R.M. ; Yu , Y. ; Xu , X.D. ; Koszyk , F.J. ; Callins , P.W. ; Kobaldt , C.M. ; Veenhuizen , A.W. ; Perkins , W.E. ; Casler , J.J. ; Masferrer , J.L. ; Zhung , Y.Y. ; Gregory , S.A. ; Seibert , K. ; Isakson , P.C. J. Med. Chem . 1997 , 40 , 1634 1647 .
- Ellis , G.W. ; Alexander , T.M.R. Chem. Rev . 1987 , 87 , 779 794 .
- Sundermeier , M. ; Zapf , A. ; Beller , M. Eur. J. Inorg. Chem . 2003 , 19 , 3513 3526 .
- Zhang , A. ; Neumeyer , J.L. Org. Lett . 2003 , 5 , 201 203 .
- Zanon , J. ; Klapars , A. ; Buchwald , S.L. J. Am. Chem. Soc . 2003 , 125 , 2890 2891 .
- Yang , C. ; Williams , J.M. Org. Lett . 2004 , 6 , 2837 2840 .
- Tamilselvan , P. ; Basavaraju , Y.B. ; Sampathkumar , E. ; Murugesan , R. Catal. Commun . 2009 , 10 , 716 719 .
- Movassagha , B. ; Shokri , S. Tetrahed. Lett . 2005 , 46 , 6923 6925 .
- Yamaguchi , K. ; Fujiwara , H. ; Ogasawara , Y. ; Kotani , M. ; Mizuno , N. Angew. Chem. Int. Ed . 2007 , 46 , 3922 3925 .
- Sarvari , M.H. Synthesis 2005 , 5 , 787 790 .
- Iranpoor , N. ; Zeynizadeh , B. Synthet. Commun . 1999 , 29 , 2747 2752 .
- Yan , P. ; Batamack , P. ; Prakash , G.K.S. ; Olah , G.A. Catal. Lett . 2005 , 101 , 141 143 .
- Meshram , H.M. Synthesis 1992 , 10 , 943 944 .
- Hegedüs , A. ; Cwik , A. ; Hell , Z. ; Horváth , Z. ; Esek , Á. ; Uzsoki , M. Green Chem . 2002 , 4 , 618 620 .
- Dewan , S.K. ; Singh , R. Synthet. Commun . 2003 , 33 , 3085 3088 .
- Lingaiah , N. ; Narender , R. Synthet. Commun . 2002 , 32 , 2391 2394 .
- Pizzio , L.R. ; Vázquez , P.G. ; Cáceres , C.V. ; Blanco , M.N. ; Alesso , E.N. ; Torviso , M.R. ; Lantanõ , B. ; Moltrasio , G.Y. ; Aguirre , J.M. App. Catal. A: Gen . 2005 , 287 , 1 8 .
- Sepúlveda , J.H. ; Yori , J.C. ; Vera , C.R. App. Catal. A: Gen . 2005 , 288 , 18 24 .
- Yadav , J.S. ; Reddy , B.V.S. ; Pandurangam , T. ; Reddy , Y.J. ; Gupta , M.K. Catal. Commun . 2008 , 9 , 1297 1301 .
- Rafiee , E. ; Shahbazi , F. J. Mol. Catal. A: Chem . 2006 , 250 , 57 61 .
- Rafiee , E. ; Shahbazi , F. ; Joshaghani , M. ; Tork , F. J. Mol. Catal. A: Chem . 2005 , 242 , 129 134 .
- Satam , J.R. ; Parghi , K.D. ; Jayaram , R.V. Catal. Commun . 2008 , 9 , 1071 1078 .
- Satam , J.R. ; Jayaram , R.V. Catal. Commun . 2008 , 9 , 1937 1940 .
- Dias , J.A. ; Caliman , E. ; Dias , S.C.L. ; Paulo , M. ; de Souza , A.T.C.P. Catal. Tdy . 2003 , 85 , 39 48 .
- Kapustyn , G.I. ; Brueva , T.R. ; Kljacko , A.L. ; Timofeyeva , M.N. ; Kulikov , S.M. ; Kozevnikov , I.V. Kinet. Katal . 1990 , 31 , 1017 1020 .
- Ghiaci , M. ; Esfahani , R.N. ; Aghaei , H. Catal. Commun . 2009 , 10 , 777 780 .
- Luche , J.L. ; Rodriguza , L.H. ; Crabbe , P. Chem. Commun . 1978 , 14 , 601 602 .
- Vazquez , P. ; Pizzio , L. ; Caceres , C. ; Blanco , M. ; Thomas , H. ; Alesso , E. ; Finkielsztein , L. ; Lantano , B. ; Moltrasio , G. ; Aguirre , J. J. Mol. Catal. A: Chem . 2000 , 161 , 223 232
Supporting Information
X-ray diffraction (XRD) study
XRD patterns of the catalysts are similar to that of the support (SiO2), lacking diffraction lines corresponding to heteropolyanions. This indicates that the acids are highly dispersed on the support surface or present as non-crystalline species.
FT-IR spectra study
By means of FT-IR, it may be observed that the spectra of the catalysts exhibit bands coinciding with those of bulk acids, although some overlapped with those of the support, which has bands at 1100, 800 and 470 cm-1. The bulk HPW spectrum shows bands at 1081, 982, 888, 793, 595 and 524 cm-1, which coincide with those referred to in the literature for the acid H3PW12O40. The first five bands are assigned to the stretching vibrations P-Oa, W-Od, W-Ob-W, W-Oc-W, and to the bending vibration Oa-P-Oa, respectively. The subscripts indicate oxygen bridging the W and the heteroatom a. corner-sharing, b. and edge-sharing oxygen, c. belonging to octahedra WO and d. terminal oxygen. HPW-SiO2 catalyst shows the bands at 982 and 793 cm-1 as an increase in transmittance of support bands, while the band at 888 cm-1 is observed with-out overlapping. (a) These results corroborate that HPW is the present on the SiO2 with an undegraded structure (Citation33).
BET surface area measurements
BET surface area of HPW and HPW-SiO2 were analyzed and it was found that HPW showed 7 m2g-1 of surface area, while HPW-SiO2 showed increase in the surface area up to 176 m2g-1.
Acidity measurements using NH3-TPD analysis
The NH3-TPD measurements revealed that negligible amount of ammonia was adsorbed on SiO2. Both the samples HPW and HPW-SiO2 showed ammonia desorption in two steps, as reported by Kapustyn et al. (Citation33). The low temperature desorption peak observed between 373 K and 403 K, indicates the presence of weak acidic sites while the high temperature desorption peak between 623 K and 673 K indicates strong acidic sites. HPW and HPW-SiO2 were found to contain 26.0 µmol/gm and 15.0 µmol/gm of strong acidic sites respectively while at low temperature HPW-SiO2 was found to contain more weak acidic sites (10 µmol/gm) than HPW (4.0 µmol/gm). However in both cases for HPW and HPW-SiO2 the number of strong acidic sites was higher than that for weak acidic sites.