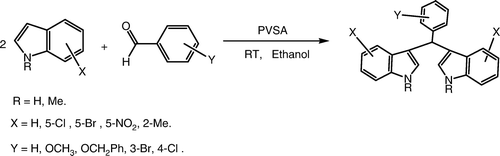
Abstract
Polyvinylsulfonic acid as a novel, biodegradable, and efficient Brønsted acid catalyst for the reaction of indoles with aldehydes to obtain bis(indolyl)methanes has been reported. The catalyst exhibited remarkable activity, recyclability, and tolerated a wide variety of functional groups providing the desired bis(indolyl)methanes in excellent yield (64–96%) at room temperature using ethanol as a solvent. The effect of different reaction parameters like solvent, temperature, catalyst doses, and recyclability were investigated for the title reaction.
Introduction
Indoles and their derivatives are valuable bioactive heterocycles useful for biological and pharmaceutical applications Citation1. For example, bis(indolyalkanes) and their derivatives are found in bioactive metabolites of terrestrial and marine origin Citation2 Citation3. Therefore, there is a great deal of interest in the synthesis of this class of compounds. Bis(indolyl)methanes are obtained by the condensation of two molecules of indoles with one molecule of aldehyde or ketone in the presence of a Lewis acid or Brønsted acid catalyst. It was observed that Lewis acid catalysts are deactivated or decomposed by the presence of nitrogen in the reactants. Hence, it necessitates having more than stoichiometric amounts of Lewis acid in the reaction mixture Citation4. A number of synthetic methods for preparation of bis(indolyl)alkane derivatives have been reported in the literature in the presence of either a Lewis acids, such as molecular iodine Citation5, lithium perchlorate Citation6, Dy(OTf)3 Citation7, TiO2 Citation8, sodium dodecylsulfonate Citation9, ZrOCl2/silica gel Citation10, polyindole salts Citation11, phosphated zirconia Citation12, and polyaniline Citation13, tungstophosphoric acid/zirconia Citation14, CeCl3·7H2O Citation15, RuIII complexes Citation16, or protic acids like glycerol as a promoter Citation17, phosphorodiamidic acid Citation18, SBA-15 supported poly(4-styrenesulfonyl(perfluorobutylsulfonyl)imide) Citation19, Brønsted acidic ionic liquids Citation20 Citation21, cellulose sulfuric acid Citation22, PEG-supported sulfonic acid Citation23, and tetrabutylammonium tribromide Citation24.
Polyvinylsulfonic acid (PVSA) is a strong aliphatic polymeric sulfonic acid and has a very high solubility in water and lower alcohols. The use of PVSA has several advantages over conventional Brønsted acid catalyst like its biodegradability, cost-effectiveness, ease of handling, recyclability, and tunable Brønsted acidity. In spite of these attractive features, it was rarely explored for various acid-catalyzed transformations. We herein report the application of PVSA as a Brønsted acid catalyst in the synthesis of bis(indolyl)methane () derivatives with only 10 mol% concentration. To the best of our knowledge, this is only second example of PVSA as a catalyst for any organic transformation Citation25.
Results and discussion
Characterization of polyvinylsulfonic acid (PVSA)
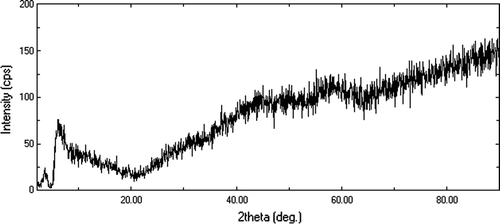
Sodium salt of polyvinylsulfonic acid (Na-PVSA), a precursor of PVSA, was characterized by XRD, DSC, and FT-IR techniques Citation25. XRD profile showed absence of sharp peaks, conforming amorphous nature of Na-PVSA. DSC thermograph showed thermal stability of Na-PVSA up to 350°C, however, peak at 92.6°C was observed due to removal of water molecule from hydrated Na-PVSA.
X-ray diffraction and DSC studies of sodium salt of polyvinylsulfonic acid (Na-PVSA)
X-ray diffraction profile was obtained and absence of any sharp peak in XRD pattern confirms the amorphous nature of Na-PVSA (). DSC thermograph of Na-PVSA shows no sharp peaks in the curve indicating its thermal stability up to 350°C; however, a peak at 92.6°C was observed due to removal of water molecule from the structure of hydrated Na-PVSA.
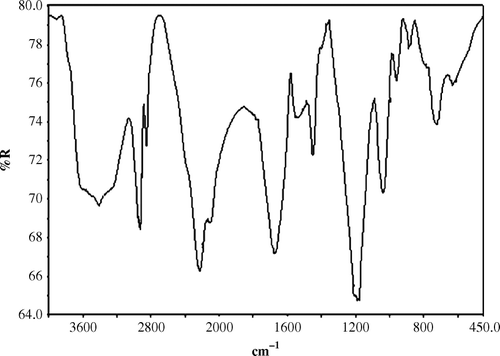
FT-IR spectrum of sodium salt of polyvinylsulfonic acid (Na-PVSA)
FT-IR spectrum of Na-PVSA () showed a medium band at 724 cm−1 due to the polymeric methylene group (−CH2−). The intense peak at 1189 cm−1 is attributed to sulfonate group. The band at 1448 cm−1 is assigned to CH2 bond deformation. The intense peak at 1673 cm−1 is attributed to in-plane deformation of water molecule. The band at 2924 cm−1 is due to CH2 stretching vibration. Stretching vibration of −OH group of water molecule is a broad band and observed at about 3441 cm−1. Thus, from the above spectra, formation of Na-PVSA is confirmed.
Optimization of reaction conditions and substrate studies
Optimization study was carried out using indole (2 mmol) and benzaldehyde (1 mmol) with variation in catalyst loading (PVSA), solvent, temperature, and time (, Entries 1–16).
Table 1. Optimization of reaction conditions of indole and benzaldehyde.a
Effect of various solvents, such as methanol, water, ethanol, acetonitrile, and chloroform were studied as shown in (Entries 1–5). Solvent-free reaction was also carried to ensure the role of solvent, which gave lower yield of 86% in comparison to the reaction with solvent ethanol (, Entry 8). Further ethanol was chosen as solvent as it can be derived from renewable sources, biodegradable, its ease of availability, and non-hazardous nature. The effect of temperature was studied at 25–28°C (room temperature [RT]), 50°C, and 80°C. The yields obtained at RT was highest, while at higher temperature were lower (, Entries 7, 10, and 11). Catalyst loading of 0 mol%, 10 mol%, 15 mol% and 20 mol% gave yields of 0%, 93%, 92% and 90%, respectively, as shown in (Entries 3, 6, 7, 9).
Based on optimized conditions in reaction of indole and benaldehyde using PVSA as catalyst, we have studied other acidic catalysts, such as PTSA, MSA, ion exhange resin (indion 225), sulfuric acid, and sulfamic acid for comparing the efficiency of PVSA (, Entries 7, 12–16). The results indicate that PVSA catalyze reaction better than other catalysts except sulfuric acid, which is highly corrosive and hazardous.
To explore the generality and scope of this method, a wide variety of substituted indoles and aldehydes were reacted using 10 mol% of PVSA under optimized experimental conditions to afford the corresponding bis(indolyl)methanes in good to excellent yields (, 64–96% yield). The products were isolated and were characterized by 1H NMR and FT-IR spectroscopy.
Table 2. PVSA catalyzed synthesis of bis(indolyl)methanes.a
In case of substituted indoles, comparable yields were obtained to that of indole (, Entries 1–3, 5, 6). The effect of orientation of substituted group in benzaldehyde ring was shown by the yields of the bis(indolyl)methanes (, Entry 1a–k). The yields of ortho-substituted benzaldehydes showed slight lowered yield, probably due to steric factors (, Entry 1f). In case of cinnamaldehyde, yields are excellent. The lower yields were obtained when N-methyl indole was reacted, in comparison with 2-methyl indole (, Entries 4, 5).
It has been reported that aromatic aldehydes with strong electron withdrawing substituents requires longer reaction time giving low to moderate yields of corresponding bis(indolyl)methanes. In this context, present protocol is note worthy because even 3-nitrobenzaldehyde underwent smooth reaction with indole giving 96% yield of bis(indolyl)methane within 2 h.
Recovery and recycle of catalyst
Under optimized reaction conditions, reaction of indole and benzaldehyde was studied for three recycle studies and it was found that catalyst was recyclable without loss in activity ().
Table 3. Catalyst recyclability.a
Experimental
General
The monomer 40% w/w sodium vinylsulfonate was procured from M/s Dharamasi Morarjee Chemical Company, India. Potassium persulfate and sodium bisulfite required for the polymer preparation were obtained from M/s S.D. Fine Chemicals, India. Indoles and aldehydes were procured from M/s Aldrich Chemicals and used without purification.
X-ray diffractograms of Na-PVSA was obtained with Siemens D-5000 XRD diffractometer. DSC of Na-PVSA was taken by Perkin-Elmer pyres 6 DSC instrument. IR spectra were recorded using Perkin-Elmer FT-IR spectrometer (100 spectrochem series). Viscosity measurement was carried out using Ubbelohde Viscometer. The reaction analysis is performed by HPLC (Jasco, Column C-8, 25 cm) with UV detector. 1H NMR spectra were recorded on Varian-400 NMR spectrometer using TMS as internal standard.
Preparation of polyvinylsulfonic acid (PVSA)
PVSA was prepared in two steps by Breslow's method and a method described in earlier published article Citation25, Citation26.
Preparation of sodium salt of polyvinylsulfonic acid (Na-PVSA)
A sample of 150 g of 40% sodium vinylsulfonate was acidified with dilute sulfuric acid to pH 4.5 and transferred to a 250-mL amber-colored glass bottle. Potassium persulfate of 0.6 g followed by 0.24 g of sodium bisulfite were added to the reaction mixture at 5°C. Reaction mass was stirred well and kept at 5°C for 24 h. The reaction mass was transferred to a beaker containing 20mL of water.. About 250 mL of methanol was added to give a viscous polymer. The polymer was then dissolved in 250 mL of water and was re-precipitated by adding 750 mL of methanol. Treating the polymer with methanol gives a gummy precipitate (56.1 g) after drying at 75°C in vacuum.
The obtained Na-PVSA was dissolved in 210 mL of water and was re-precipitated by addition of 100 mL methanol. The oily product appeared was separated and was dissolved in 80 mL water and was re-precipitated with addition of 40 mL of methanol. After separating, the oil was again treated with 200 mL of methanol. Drying the product at 75°C under vacuum gave 34 g of Na-PVSA product. The specific viscosity was found out to be 1.72 indicating its average molecular weight of ∼55,000 which is relevant to the reported one.
Preparation of polyvinylsulfonic acid (PVSA)
Na-PVSA (32 g) was dissolved in 500 mL of distilled water. The solution was passed through a cation exchange column containing 250 mL activated cation exchange resin (Indion 225, capacity = 1.8 meq mL−1). The column was then washed with distilled water till the eluent showed absence of acidic pH. The collected acidic fraction was concentrated in vacuum at 80°C to give 16 g PVSA. Distilled water (16 mL) was then added and stirred well till clear solution obtained. This solution contains ∼50% w/w PVSA in water. PVSA concentration in solution was measured by acidimetric titration. This solution was used as a catalyst for our entire study. The pH of 0.01N solution was checked and was found to be 2.83.
Typical procedure for preparation of 3,3′-bis(indolyl)phenylmethane
Indole (234 mg, 2 mmol) and benzaldehyde (106 mg, 1 mmol) were added to 1 mL of absolute ethanol containing 21.6 mg (0.1 mmol) of PVSA (50% w/w). The reaction mixture was stirred for 2 h at RT (25–28°C). The progress of the reaction was monitored by TLC. After 2 h, 10 mL of ethyl acetate and 5 mL of water was added to the reaction mixture. The product was extracted in ethyl acetate layer, washed with small quantity of water, dried over sodium sulfate, and evaporated under vacuum to give crude product. The crude solid product obtained was analyzed by HPLC using acetonitrile:water (60:40) and was purified using column chromatography by eluting with petroleum ether–ethyl acetate solvent system. All the compounds are well known and their typical characterization data are reported Citation6, Citation15–18 Citation21, Citation24.
Spectral data for selected products
3,3′-Bis(indolyl)phenylmethane. IR (KBr): 3478, 3019, 1601, 1522, 1456, 1419, 1215, 1093, 1017, 757, 669 cm−1; 1H NMR (CDCl3): δH 5.89 (1H, s), 6.67 (2H, s), 7.09–7.58 (13H, m), 7.94 (2H, bs, NH) (, Entry 1a).
3,3′-Bis(indolyl)-3,4 dimethoxyphenylmethane. IR (KBr): 3480, 3020, 1604, 1512, 1456, 1418, 1336, 1216, 1091, 1033, 759 cm−1; 1H NMR (CDCl3): δH 3.76 (3H, s), 3.85 (3H, s), 5.83 (1H, s), 6.65 (2H, d), 6.78 (2H, d), 7.0 (3H, t), 7.17 (2H, t), 7.29–7.43 (4H, m), 7.91 (2H, bs, NH) (, Entry 1k).
3,3′-Bis(indolyl)-4-chlorophenylmethane. IR(KBr): 3478, 3020, 2927, 1600, 1523, 1456, 1417,1216, 1091,1015, 759, 670 cm−1; 1H NMR (CDCl3): δH 5.88 (1H, s), 6.63 (2H, brs), 7.00–7.70 (12H, bs, NH) (, Entry 1c).
Recovery and recycle of catalyst
Filtrate and washings of the reaction mixture were combined and were passed through an activated cation exchange resin (indion 225) column. Distilled water washings were given till pH was acidic. Collected fraction was evaporated to dryness in rotary evaporator. The content of the flask was dissolved in appropriate quantity of dry ethanol and was used in recycle experiment.
Conclusion
In summary, we have demonstrated that PVSA is an excellent catalyst for the reaction of indoles and aromatic aldehydes to give bis(indolyl)methanes. High activity and easy handling makes PVSA an ideal catalyst for this transformation. The procedure has the advantages of mild reaction conditions, high yields of products, short reaction time, recycleability, and simple experimental technique, making it a useful and attractive process for the synthesis of bis(indolyl)methane derivatives.
References
- Sundberg , R.J. Chemistry of Indoles ; New York : Academic Press , 1996 .
- Porter , J.K. ; Bacon , C.W. ; Robin , J.D. ; Himmelsbach , D.S. ; Hingman , H.C. J. Agric. Food Chem . 1977 , 25 , 88 93 .
- Osawa , T. ; Namiki , M. Tetrahedron Lett. 1983 , 24 , 4719 4722 .
- Kobayashi , S. ; Arki , M. ; Yasda , M. Tetrahedron Lett . 1995 , 36 , 5773 5776 .
- Bandger , B.P. ; Shaikh , K.A. Tetrahedron Lett . 2003 , 44 , 1959 1961 .
- Yadav , J.S. ; Subba Reddy , B.V. ; Murthy , C.V.S.R. ; Mahesh Kumar , G. ; Madan , C. Synthesis 2001 , 5 , 783 787 .
- Yulding , M. ; Sanzhong , L. ; Jiagi , H. ; Jin-Pei , C. Tetrahedron Lett . 2004 , 45 , 4567 4570 .
- Hossenni-Sarvan , M. Acta Chim. Slov . 2007 , 54 , 354 359 .
- Deb , M.L. ; Bhuyan , J. Tetrahedron Lett . 2006 , 47 , 1441 1443 .
- Firozabadi , H. ; Iranpoor , N. ; Jafarpour , M. ; Ghaderi , A. J. Mol. Catal. A Chem . 2006 , 253 , 249 251 .
- Palaniappan , S. ; John , A. J. Mol. Catal. A Chem . 2005 , 242 , 168 172 .
- Nadkarni , S.V. ; Gawande , M.B. ; Jairam , R.V. ; Nagarkar , J.M . Catal. Commun . 2008 , 9 , 1728 1733 .
- Palaniappan , S. ; Saravanan , C. ; Amarnath , C.A. ; Rao , V.J. Catal. Lett . 2004 , 97 , 77 81 .
- Satam , J.R. ; Parghi , K.D. ; Jayaram , R.V. Catal. Commun . 2008 , 9 , 1071 1078 .
- Silveira , C.R. ; Mendes , S.R. ; Líbero , F.M. ; Lenardão , E.J. ; Perin , G. Tetrahedron Lett . 2009 , 50 , 6060 6063 .
- Tabatabaeian , K. ; Mamaghani , M. ; Mahmoodi , N. ; Khorshidi , A. Can. J. Chem . 2006 , 84 , 1541 1545 .
- He , F. ; Li , P. ; Gu , Y. ; Li , G. Green Chem . 2009 , 11 , 1767 1773 .
- He , Q.L. ; Sun , F.L. ; Zheng , X.J. ; You , S.L. Synlett 2009 , 7 , 1111 1114 .
- Ma , Z.-H. ; Han , H.-B. ; Zhou , Z.-B. ; Nie , J. J. Mol. Catal. A Chem . 2009 , 311 , 46 53 .
- Sadaphal , S.A. ; Shelke , K.F. ; Sonar , S.S. ; Shingare , M.S. Cent. Eur. J. Chem . 2008 , 6 , 622 626 .
- Hagiwara , H. ; Sekifuji , M. ; Hoshi , T. ; Qiao , K. ; Yokoyama , C. Synlett 2007 , 8 , 1320 1322
- Sadaphal , S.A. ; Sonar , S.S. ; Ware , M.N. ; Shingare , M.S. Green Chem. Lett. Rev . 2008 , 1 , 191 196 .
- Sheng , S.R. ; Wang , Q.-Y. ; Ding , Y. ; Liu , X.L. ; Cai , M.Z. Catal. Lett . 2009 , 128 , 418 422 .
- Lin , X.F. ; Cui , L.S. ; Wang , Y.G. Synth. Commun . 2006 , 36 , 3153 3160 .
- Ekbote , S.S. ; Panda , A.G. ; Bhor , M.D. ; Bhanage , B.M. Catal. Commun . 2009 , 10 , 1569 1573 .
- Breslow , D.S. ; Kutner A. J. Polym. Sci . 1958 , 27 , 295 297 .