Abstract
An improved green Knoevenagel condensation of various aromatic aldehydes with 4-thiazolidinones in the presence of anhydrous ammonium acetate can be achieved by grinding at room temperature in the absence of solvents. This process is simple, efficient, economical, and environmentally benign compared to classical reactions.
Green chemistry focuses on research that attempts to reduce or eliminate negative environmental impacts. Among the aims of green chemistry Citation1 are the prevention of wastes and the generation of substances with little or no toxicity to humans and the environment, in order to maximize atom economy. This is achieved by assuring that the final product contains the maximum proportion of the starting materials and by avoiding the use of harmful solvents or, even better, not using solvents at all.
4-Thiazolidinones exhibit a variety of pharmacological activities, functioning, for example, as antibacterial Citation2–5, antifungal Citation6, antidiabetic Citation7, antitubercular Citation8 Citation9, anti-HIV Citation10 Citation11, antiparasitic Citation12, hypnotic Citation13, and anathematic agents Citation14. Also, 5-arylidene derivatives of 4-thiazolidinones have been found to be better fungistatic agents than the parent 4-thiazolidinones Citation15 Citation16. Several methods have been developed for the preparation of 5-arylidene derivatives of 4-thiazolidinones. The most common is Knoevenagel condensation between aromatic aldehydes and 4-thiazolidinones carried out in glacial acetic acid containing anhydrous sodium acetate Citation17–21. Instead of sodium acetate, acetic anhydride Citation22 Citation23, ethanolamine Citation24 Citation25, and ammonium chloride in ammonia Citation26–28 have also been used. Moreover, other derivatives were prepared by condensation of 2-thioxo-4-thiazolidinone with pyridine aldehydes using glycine and sodium carbonate as catalysts Citation29. More recent methods for the preparation of 5-benzylidene-2,4-thiazolidinediones and 5-benzylidene-2-thioxothiazolidin-4-ones have been reported that involve Knoevenagel condensation of aromatic aldehydes with 2-thioxothiazolidin-4-one or thiazolidine-2,4-dione catalyzed by a basic functionalized ionic liquid, 1-butyl-3-methylimidazolium hydroxide ([bmim][OH]) Citation30 Citation31. Also, Knoevenagel condensation under solvent-free microwave irradiation was applied to the synthesis of 5-arylidene-4-thiazolidinones Citation32. A number of 5-arylidene-2-thioxo-4-thiazolidinone derivatives were synthesized by condensation of aromatic aldehydes with 2-thioxo-4-thiazolidinone using tetrabutylammonium bromide (TBAB) as a phase transfer catalyst in water under microwave irradiation Citation33. However, in spite of their utility, some methods suffer disadvantages like long reaction times (3–6 h) with low yields, chemical hazards, and environmental pollution.
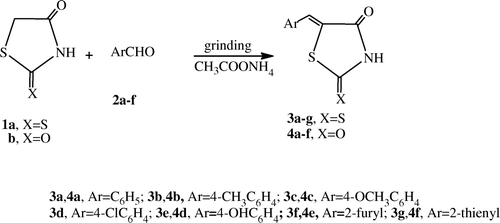
Toda et al. Citation34 have reported that many reactions can be conducted with a high yield just by grinding solids (or liquid and solids) together. As a continuation of our program to develop facile methods to synthesize 4-thiazolidinone derivatives Citation35–41, and as part of our recent interest in solid state synthesis Citation42, we report herein a simple and highly efficient method for the synthesis of some 5-arylidene-4-thiazolidinones. The reaction was carried out by grinding together equivalent amounts of the appropriate aldehydes and 4-thiazolidinones in the presence of solid anhydrous ammonium acetate in a porcelain mortar, under solvent-free conditions. Grinding for about 13–25 min led to a colored solid mass of the crude product [the completion of the reaction was checked by TLC (petroleum ether/ethyl acetate, 5:1)]. Purification was carried out by simple Buchner filtration, washing with cold water and crystallizing from an appropriate solvent to give the respective 5-arylidene-4-thiazolidinones 3a–f and 4a–e ( and Experimental).
This method is simple and effective in terms of its short reaction time, excellent yields, and the formation of single products. Comparing the preparation, for example, of 5-(4-hydroxyphenyl)methylene-2-thioxothiazolidin-4-one (3e) from 4-hydroxybenzaldehyde (2e) and 2-thioxothiazolidin-4-one (1a), by heating in acetic acid containing anhydrous sodium acetate (conventional method) or by grinding with ammonium acetate without solvent (see Experimental), it is evident that a higher yield (97%) and shorter reaction time (10 min) are obtained by the latter (see Experimental). The grinding method is consistent with the green chemistry approach also because it takes place at room temperature and avoids heat or microwave irradiation.
Experimental
Melting points were determined on an Electrothermal (9100) apparatus and are uncorrected. The IR spectra were recorded as KBr pellets on a Perkin Elmer 1430 spectrophotometer. 1H NMR spectra and 13C NMR were recorded in deuterated dimethylsulfoxide at 300 MHz on a Varian Gemini NMR spectrometer using tetramethylsilane as an internal reference, and results are expressed as δ values. Mass spectra were taken on a Shimadzu GCMS-QP 1000 Ex mass spectrometer at 70 eV. Elemental analyses were carried out at the Microanalysis Center of Cairo University, Giza, Egypt.
Conventional method for the synthesis of 5-(4-hydroxybenzylidene)-2-thioxothiazolidin-4-one (3e)
A mixture of 2-thioxothiazolidin-4-one (1a) (1.33 g, 0.01 mole) and 4-hydroxybenzaldehyde (2e) (1.22 g, 0.01 mole) was dissolved in glacial acetic acid (20 mL) and anhydrous sodium acetate (0.82 g, 0.01 mole). The mixture was refluxed for 2 h until no more starting material was detected by TLC (petroleum ether/ethyl acetate, 5:1). The separated solid was filtered and recrystallized from glacial acetic acid to give compound 3e (1.5 g, 85%), identical in all respects with an authentic sample.
General procedure
A mixture of the appropriate 4-thiazolidinone 1a,b (0.05 mole), aromatic aldehydes 2a–g (0.05 mole), and anhydrous ammonium acetate (0.574 g, 0.07 mole) was thoroughly ground with a pestle in an open mortar at room temperature for 2–3 min until the mixture turned into a melt. The initial syrupy reaction mixture solidified within 3–5 min. Grinding continued for 5–10 min while the reaction was monitored by TLC (petroleum ether/ethyl acetate, 5:1). The collected solid was washed with cold water to remove the ammonium acetate and crystallized from the appropriate solvent (glacial acetic acid or ethanol) to give the corresponding 5-arylidene-4-thiazolidinones 3a–g and 4a–f, respectively.
5-Benzylidene-2-thioxothiazolidin-4-one (3a)
Yellow crystals (AcOH), yield 0.93 g (85%), mp 204°C, νmax/cm−1 (KBr) 3130 (NH), 1672 (CO); 1H NMR (DMSO) δ=7.48–7.80 (m, 5H, Ar.), 7.70 (s, 1H, CH), 10.22 (s, 1H, NH); m/z=221.30. Anal. Calcd. C10H7NOS2: C, 54.27; H, 3.19; N, 6.33; S, 28.98. Found: C, 54.44; H, 3.0; N, 6.11; S, 28.82.
5-(4-Methylbenzylidene)-2-thioxothiazolidin-4-one (3b)
Yellow crystals, yield 0.97 g (83%), mp 233°C (AcOH), νmax/cm−1 (KBr) 3134 (NH), 1680 (CO); 1H NMR (DMSO) δ=2.35 (s, 1H, CH3), 7.35 (d, 2H, J=8.1 Hz, Ar.), 7.61 (d, 2H, J=8.1 Hz, Ar), 7.73 (s, 1H, CH), 10.83 (br., 1H, NH); m/z=235.33. Anal. Calcd. C11H9NOS2: C, 56.14; H, 3.85; N, 5.95; S, 27.25. Found: C, 56.32; H, 3.68; N, 6.17; S, 27.43.
5-(4-Methoxybenzylidene)-2-thioxothiazolidin-4-one (3c)
Yellow crystals, yield 1.1 g (85%), mp 244°C, νmax/cm−1 (KBr) 3146 (NH), 1708 (CO); 1H NMR (DMSO) δ=3.82 (s, 3H, OCH3), 7.18 (d, 2H, J=8.4 Hz, Ar.), 7.61 (d, 2H, J=8.4 Hz, Ar), 7.70 (s, 1H, CH), 11.65 (br., 1H, NH); 13C NMR (DMSO) δ=55.5, 115.0, 122.1, 125.4, 131.8, 132.6 161.2, 169.3, 195.4; m/z=251.32. Anal. Calcd. C11H9NO2S2: C, 52.57; H, 3.61; N, 5.57; S, 25.52. Found: C, 52.41; H, 3.44; N, 5.60; S, 25.69.
5-(4-Chlorobenzylidene)-2-thioxothiazolidin-4-one (3d)
Yellow crystals, yield 1.0 g (84%), mp 241°C, νmax/cm−1 (KBr) 3212 (NH), 1688 (CO); 1H NMR (DMSO) δ=7.37 (d, 2H, J=7.8 Hz, Ar.), 7.71 (s, 1H, CH), 7.97 (d, 2H, J=7.8 Hz, Ar), 11.85 (br., 1H, NH); m/z=255.74. Anal. Calcd. C10H6ClNOS2: C, 46.96; H, 2.36; Cl, 13.86; N, 5.48; S, 25.08. Found: C, 46.78; H, 2.53; N, 5.26; S, 25.25.
5-(4-Hydroxybenzylidene)-2-thioxothiazolidin-4-one (3e)
Yellow crystals, yield 1.3 g (97%), mp 310°C, νmax/cm−1 (KBr) 3372 (OH), 3088 (NH), 1689 (CO); 1H NMR (DMSO) δ=7.08 (d, 2H, J=8.1 Hz, Ar.), 7.56 (d, 2H, J=8.1 Hz, Ar), 8.05 (s, 1H, CH), 10.15 (s, 1H, OH), 12.11 (br., 1H, NH); m/z=237.30. Anal. Calcd. C10H7NO2S2: C, 50.61; H, 2.97; N, 5.90; S, 27.03. Found: C, 50.41; H, 2.79; N, 6.13; S, 27.20.
5-(Furan-2-ylmethylene)-2-thioxothiazolidin-4-one (3f)
Brownish yellow crystals, yield 0.96 g (87%), mp 232°C; νmax/cm−1 (KBr) 3437 (NH), 1685 (CO); 1H NMR (DMSO) δ=6.87–8.17 (m, 4H, furan & CH), 9.80 (br., 1H, NH); m/z=211.26. Anal. Calcd. C8H5NO2S: C, 45.48; H, 2.39; N, 6.63; S, 30.36. Found: C, 50.41; H, 2.79; N, 6.13; S, 27.20.
5-(Thiophen-2-ylmethylene)-2-thioxothiazolidin-4-one (3g)
Yellow crystals, yield 0.96 g (85%), mp 230°C, νmax/cm−1 (KBr) 3436 (NH), 1687 (CO); 1H NMR (DMSO) δ=7.65 (s, 1H, CH), 7.68–8.10 (m, 3H, thiophene), 9.94 (br., 1H, NH); m/z=227. Anal. Calcd. C8H5NOS3: C, 42.27; H, 2.22; N, 6.16; S, 42.32. Found: C, 42.13; H, 2.06; N, 6.30; S, 42.44.
5-Benzylidene-2,4-thiazolidinedione (4a)
Yellow crystals, yield 0.98 g (96%), mp 240°C, νmax/cm−1 (KBr) 3213 (NH), 1684 (CO); 1H NMR (DMSO) δ=7.05–7.86 (m, 5H, Ar.), 7.80 (s, 1H, CH), 12.57 (s, 1H, NH); 13C NMR (DMSO) δ=122.2, 130.1, 131.5, 132.7, 137.2, 140.5, 166.8, 168.1; m/z=235.26. Anal. Calcd. C11H9NO3S: C, 56.16; H, 3.86; N, 5.95; S, 13.63. Found: C, 56.35; H, 3.68; N, 5.72; S, 13.82.
5-(4-Methylbenzylidene)-2,4-thiazolidinedione (4b)
Yellow crystals, yield 1.0 g (92%), mp 232°C, νmax/cm−1 (KBr) 3213 (NH), 1684 (CO); 1H NMR (DMSO) δ=2.37 (s, 3H, CH3), 7.05 (m, 2H, J=7.8 Hz, Ar.), 7.49 (m, 2H, 7.8 Hz, Ar), 7.73 (s, 1H, CH), 12.57 (s, 1H, NH); m/z=235.26. Anal. Calcd. C11H9NO3S: C, 56.16; H, 3.86; N, 5.95; S, 13.63. Found: C, 56.35; H, 3.68; N, 5.72; S, 13.82.
5-(4-Methoxybenzylidene)-2,4-thiazolidinedione (4c)
Yellow crystals, yield 1.1 g (90%), mp 215°C, νmax/cm−1 (KBr) 3213 (NH), 1684 (CO); 1H NMR (DMSO) δ=3.81 (s, 3H, OCH3), 7.05 (m, 2H, J=8.1 Hz, Ar.), 7.49 (m, 2H, J=8.1 Hz, Ar), 7.76 (s, 1H, CH), 12.57 (s, 1H, NH); m/z=235.26. Anal. Calcd. C11H9NO3S: C, 56.16; H, 3.86; N, 5.95; S, 13.63. Found: C, 56.35; H, 3.68; N, 5.72; S, 13.82.
5-(4-Hydroxybenzylidene)-2,4-thiazolidenedione (4d)
Pale yellow crystals, yield 1.0 g (96%), mp 322°C, νmax/cm−1 (KBr) 3213 (NH), 1684 (CO); 1H NMR (DMSO) δ=3.66 (s, 1H, OH), 7.05 (d, 2H, J=8.4 Hz, Ar.), 7.51 (d, 2H, J=8.4 Hz, Ar), 7.78 (s, 1H, CH), 12.43 (s, 1H, NH); m/z=221.23. Anal. Calcd. C10H7NO3S: C, 54.29; H, 3.19; N, 6.33; S, 14.49. Found: C, 54.47; H, 3.36; N, 6.55; S, 14.31.
5-(Furan-2-ylmethylene)-2,4-thiazolidenedione (4e)
Brownish yellow crystals, yield 0.94 g (96%), mp 222°C, νmax/cm−1 (KBr) 3213 (NH), 1680 (CO); 1H NMR (DMSO) δ=6.92 (m, 1H, furan), 7.57 (s, 1H, CH), 7.70 (d, 2H, furan), 8.23 (d, 2H, furan), 12.32 (s, 1H, NH), m/z=195.20. Anal. Calcd. C8H5NO3S: C, 49.23; H, 2.58; N, 7.18; S, 16.43. Found: C, 49.42; H, 2.76; N, 7.40; S, 16.60.
5-(Thiophen-2-ylmethylene)-2,4-thiazolidenedione (4f)
Brownish yellow crystals, yield 1.1 g (94%), mp 222°C, νmax/cm−1 (KBr) 3213 (NH), 1680 (CO); 1H NMR (DMSO) δ=6.92 (m, 1H, furan), 7.57 (s, 1H, CH), 7.70 (d, 2H, furan), 8.23 (d, 2H, furan), 12.32 (s, 1H, NH), m/z=195.20. Anal. Calcd. C8H5NO3S: C, 49.23; H, 2.58; N, 7.18; S, 16.43. Found: C, 49.42; H, 2.76; N, 7.40; S, 16.60.
References
- Anastas , P. and Warner , J. 1998 . Green Chemistry: Theory and Practice , New York : Oxford University Press .
- Gualtieri , M. ; Bastide , L. ; Latouche , P.V. ; Leonette , J.P. J. Antimicrob. Chemother. 2006 , 58 , 778 – 783 .
- Sim , M.M. ; Ng , S.B. ; Buss , A.D. ; Crasta , S.C. ; Goh , K.L. ; Lee , S.K. Bioorg. Med. Chem. Lett. 2002 , 12 , 697 – 699 .
- Dündar , O.B. ; Özgen , O. ; Menteşe , A. ; Altanlar , N. ; Atl , O. ; Kendi , E. ; Ertan , R. Bioorg. Med. Chem. Lett. 2007 , 15 , 6012 – 6017 .
- Gouveia , F.L. ; de Oliveira , R.M.B. ; de Oliveira , T.B. ; da Silva , I.M. ; do Nascimento , S.C. ; de Seua , K.X.F.R. ; de Albuquerque , J.F.C. Eur. J. Med. Chem. 2009 , 44 , 2038 – 2043 .
- Sortino , M. ; Delgado , P. ; Juarez , S. ; Quiroqa , J. ; Abonia , R. ; Insuasty , B. ; Noqueras , M. ; Rodero , L. ; Garibatto , F.M. ; Enriz , R.D ; Zacchino , S.A. Bioorg. Med. Chem. 2007 , 15 , 484 – 494 .
- Nurugan , R. ; Anbazhagan , S. S. , Narayanan , S. Eur. J. Med. Chem. 2009 , 44 , 3272 – 3279 .
- Chandrappa , S. ; Kavitha , C.V. ; Shahabuddin , M.S. ; Vinaya , K. ; Ananda , K.C.S. ; Ranganatha , S.R. ; Raghavan , S.C. ; Rangappa , K.S. Bioorg. Med. Chem. 2009 , 17 , 2576 – 2584 .
- Brooke , E.W. ; Davies , S.G. ; Mulvaney , A.W. ; Okada , M. ; Pompeo , F. ; Sim , E. ; Vickers , R. J. ; Westwood , I.M. Bioorg. Med. Chem. Lett. 2003 , 13 , 2527 – 2530 .
- Ozkirimli , S. ; Kazan , F. ; Tunali , Y. J. Enzyme Inhib. Med. Chem. 2009 , 24 , 447 – 452 .
- Chandrappa , S. ; Benaka Prasad , S.B. ; Vinaya , K. ; Ananda , K.C.S. ; Thimmegowda , N.R. ; Rangappa , K.S. Invest. New Drugs 2008 , 26 , 437 – 444 .
- Rauter , A.P. ; Padilha , M. ; Figueiredo , J.A. ; Ismael , M.I. ; Justino , J. ; Ferreira , H. ; Ferreira , M.J. ; Rajendran , C. ; Wilkins , R. ; Vaz , P.D. ; Calhorda , M.J. J. Carbohydr. Chem. 2005 , 24 , 275 – 296 .
- Ergenc , N. ; Capan , G. ; Gunay , N.S. ; Ozkirimli , S. ; Gungor , M. ; Ozbey , S. ; Kendi , E. Arch. Pharm. 1999 , 332 , 343 – 347 .
- Verma , A. ; Saraf , S.K. Eur. J. Med. Chem. 2008 , 43 , 897 – 905 .
- Ottona , R. ; Maccari , R. ; Barreca , M.L. ; Bruno , G. ; Rotondo , A. ; Rossi , A. ; Chiricosta , G. ; Dipaola , R. ; Sautebin , L. ; Cuzzocrea , S. ; Vigorita , M.G. Bioorg. Med. Chem. 2005 , 13 , 4243 – 4252 .
- Ameya , A.C. ; Nandini , R.P. Arkivoc (xvi) 2007 , 14 , 148 – 155 .
- Brown , F.C. , Bradsher , C.K. ; Bond , S.M. Ind. Eng. Chem. 1953 , 45 , 1030 .
- Taniyama , H. ; Yasui , B. J. Pharm. Soc. Japan 1955 , 75 , 200 .
- Allan , F. ; Allan , G. ; Thomson J. J. Org. Chem. 1958 , 23 , 112 – 116 .
- Baranov , S.B. ; Komoritsa , L.D. Khim. Geterotsikl. Soedin. 1965 , 1 , 69 .
- Oya Bozdag , O. ; Kilclgil , G.A. ; Tuncbilek , M. ; Ertan , R. Turk. J. Chem . 1999 , 23 , 163 – 170 .
- Yarovenko , V.N. ; Nikitina , A.S. ; Zavarzin , I.V. ; Krayushkin , M.M. ; Kovalenko , L.V. Russ. Chem. Bull . 2007 , 56 , 1624 – 1630 .
- Irvine , M.W. ; Patrick , G.L. ; Kewney , J. ; Aastings , S.F. ; Mackenzie , S.J. Bioorg. Med. Chem. Lett . 2008 , 18 , 2032 – 2037 .
- Iwao , J. ; Tomino , K. J. Pharm. Soc. Japan 1956 , 76 , 755 .
- Marton , J. ; Enisz , J. ; Hosztafi , S. ; Tmar T. J. Agric Food Chem . 1993 , 41 , 148 – 152 .
- Alekseenko , B.A. ; Gorizdra , T.E. ; Baranov , S.N. Khim. Geterotsikl. Soedin . 1969 , 5 , 230 – 231 .
- Allan , G.G. ; Duncan , M. ; Newbold , G.T. J. Chem. Soc . 1952 , 5053 – 5055 .
- Brown , F.C. ; Bradsher , C.K. ; McCallum , S.G. ; Pother , M. J. Org. Chem . 1950 , 15 , 174 – 176 .
- Chowdhy , M.M. ; Michael , D. ; Mingas , P. ; White , A.J.P. ; William , D.J. J. Chem. Soc., Perkin Trans . 1 2000 , 3495 – 3504 .
- Gong , K. ; Wei , H.Z. ; Ku , Y. ; Fang , D. ; Liang , L.Z. Monatsh. Chem . 2008 , 139 , 913 – 915 .
- Shelke , K.F. ; Sapkal , S.B. ; Madja , B.R. ; Shingate , B.B. ; Shingare , M.S. Bull. Catal. Soc . 2009 , 8 , 30 – 34 .
- Zhou , J.F. ; Song , Y.Z. ; Zhu , F.X. ; Zhu , Y.L. Synth. Commun . 2006 , 36 , 3297 – 3303 .
- Bourahla , K. ; Derdour , A. ; Rahmouni , M. ; Carreaux , F. ; Bazureau , J.P. Tetrahedron Lett . 2007 , 48 , 5785 – 5789 .
- Toda , F. ; Tanka , K. ; Sekikawa , A. J. Chem. Soc. Chem. Commun . 1987 , 279 – 280 .
- Abddallah , S.O. ; Hammoude , H.A. ; Metwally , N.H. Egypt J. Chem . 1985 , 28 , 497 – 503 .
- Ead , H.A. ; Abdallah , S.O. ; Kassab , N.A. ; Metwally , N.H. ; Saleh , Y.E. Arch. Pharm. (Weinheim) 1987 , 320 , 1227 – 1232 .
- Ead , H.A. ; Metwally , N.H. Arch. Pharm. (Weinheim) 1990 , 323 , 57 – 58 .
- Metwally , N.H. Indian J. Chem . 2000 , 39B , 757 – 763 .
- Metwally , N.H. Phosphorus, Sulfur, Silicon Relat. Elem . 2008 , 183 , 34 – 43 .
- Metwally , N.H. Heterocycles 2008 , 75 319 – 329 .
- Metwally , N.H. Phosphorus, Sulfur, Silicon Relat. Elem . 2008 , 183 , 2073 – 2085
- Rateb , N.M. ; Zohdi , H.F. Synth. Commun . 2009 , 39 , 2784 – 2789 .