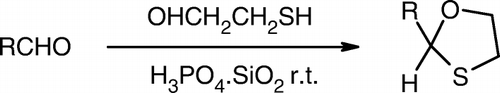Abstract
A silica supported orthophosphoric acid catalyst (H3PO4. SiO2) was prepared by stirring silica gel (100–200mesh) with orthophosphoric acid in chloroform at room temperature. The catalyst was characterized by scanning electron microscopy (SEM) and energy dispersion analytical X-ray (EDX). It demonstrated excellent activity, chemoselectivity, and recyclability for oxathioacetalization of aldehydes.
Introduction
The growing awareness of environmentally friendly approaches in organic synthesis has been influenced by the use of solid acids and chemical processes on solid surfaces. In general, heterogeneous catalysis is preferred over homogeneous catalysis in industrial processes as the extraction of the product and recovery of the catalyst are relatively easier Citation1–4. The development of non-metallic solid catalysts has drawn much attention in recent times due to their advantage of minimal product contamination from metal leaching. Apart from this, because of their ease of preparation, efficiency, and reusability, silica-supported catalysts as well as various acids impregnated on silica gel have gained considerable attention. So far various silica supported catalysts have been reported, e.g. SOCl2/SiO2 Citation5, CoBr2/SiO2 Citation6, ZrCl4/SiO2 Citation7, TaCl5/SiO2 Citation8, Cu(OTf)2/SiO2 Citation9, HClO4/SiO2 Citation10, etc. The versatility and overall synthetic utility of such silica supported acid catalysts can be exemplified by their applications to various organic transformations such as stereoselective synthesis of thiochromans Citation11; acetylation of phenols, alcohols, and amines Citation12; glycosylation Citation13; synthesis of quinolines Citation14; etc. Fay et al. Citation15 used TEMPO supported on silica for Anelli oxidation of alcohols. Dias et al. Citation16 reported metal oxide supported on silica as an efficient catalyst for oxidation of methanol to formaldehyde. In one of the recent reports of esterification of carboxylic acids, Chakraborty et al. Citation17 used silica supported perchloric acid. However, most of these catalysts have one or more drawbacks such as being difficult to prepare, sensitive to moisture, corrosive, etc. Also, silica supported metal catalysts such as CoBr2/SiO2, ZrCl4/SiO2, Cu(OTf)2/SiO2, and AlCl3/SiO2 may lead to contamination of products with trace amounts of metal, which is one of the major problems in synthesis of pure active pharmaceutical ingredients (APIs) and industrially important compounds.
Today, organic synthesis has reached a remarkable level of competence, and even the most complex molecules are accessible. The prerequisites for this success are both the availability of a wide range of efficient synthetic methods and reagents. However, the complex synthetic intermediates and products contain, in general, a multiplicity of functional groups, most of which must be blocked and, at an appropriate point in the synthesis, liberated. In multistep synthesis of various industrially important polyfunctional organic molecules, the protection of carbonyl groups plays a key role. In this paper we report an easy-to-prepare, cost-effective, efficient, and reusable silica supported orthophosphoric acid catalyst for the protection of aldehydes as oxathioacetals.
Results and discussion
Catalyst synthesis and characterization
As a part of our program toward the development of environmentally benign protocols, herein we report a silica supported orthophosphoric acid catalyst for oxathioacetalization of aldehydes. Even though a similar kind of silica supported polyphosphoric acid catalyst has been reported earlier Citation18, the present catalyst has the advantage of chemoselectivity () over the reported catalyst. We have also successfully characterized this silica supported catalyst with the help of scanning electron microscopy (SEM) and energy dispersion analytical X-ray (EDX).
In a typical experiment, taking the reference of earlier reports Citation11 Citation18 Citation19 of preparation of silica supported catalysts, the orthophosphoric acid was added to a stirred suspension of silica gel in chloroform. After stirring for 2h the white silica turned gray, indicating the formation of the catalyst. Then the chloroform was evaporated in vacuo, and the resultant powder was dried at 100°C to give a free-flowing material as catalyst.
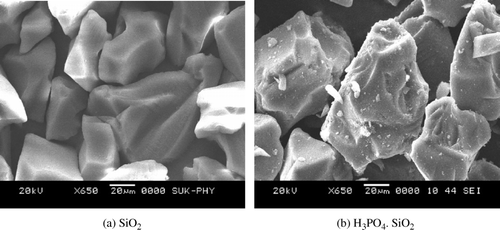
To study the surface morphology of the catalyst, the SEM monographs of the catalyst prepared were compared with that of the silica gel used for its preparation. The SEM images of the catalyst () showed small buds on the surface. This change in surface morphology prompted us to go for EDX analysis of the catalyst. In EDX analysis (see supporting information) the characteristic peak at 2.013keV for phosphorous confirmed the presence of phosphorous. The percentage mass content of phosphorous (15.83) perfectly matched the theoretically calculated value (15.82). This particular information revealed that the orthophosphoric acid had become supported on the silica.
Application of catalyst for oxathioacetalization
There are various reports Citation19–22 on protection of carbonyl compounds by acetalization, thioacetalization, oxathioacetalization, and dithioacetalization. However, the oxathioacetalization has advantages like stability in acidic conditions and easy removal. Aoyama et al. Citation18 used PPA.SiO2 for oxathioacetalization of aldehydes and ketones. In one of the recent reports Kumar et al. Citation23 used nanosized nickel particles for selective protection of carbonyl compounds. In their report of oxathioacetalization of carbonyl compounds, Liang et al. Citation24 used a novel organo catalyst. Recently, Konwar et al. Citation25 demonstrated the application of tin (IV) hydrogen phosphate nanodiscs for oxathioacetalization of aldehydes.
Further, to test the utility of the catalyst in organic transformations, we decided to use the catalyst for oxathioacetalization of aldehydes (). Taking benzaldehyde as the model substrate, we investigated the reaction in various solvents as indicated in . The reaction proceeded with good yields in chloroform. An attempt to further improve the method and yield prompted us to try solvent-free conditions, and to our delight the reaction went smoothly under solvent-free conditions. To explore the efficiency of the silica supported orthophosphoric acid catalyst and to optimize the reaction conditions, a set of reactions were carried out under different catalytic conditions taking benzaldehyde as the model substrate. It was found that there was no reaction without the catalyst or in the presence of silica even after stirring at room temperature for 20h. We studied the reaction using 50, 25, and 20mg of catalyst per mmol of substrate and found that the reaction proceeded smoothly with good yields in the presence of 25mg of catalyst per mmol. There was no any substantial effect on yields even after use of 50mg of catalyst.
Table 1. Table 1. Optimization of reaction conditions.
It was found that a number of aromatic and aliphatic aldehydes underwent the reaction smoothly under solvent-free conditions at room temperature with good to excellent yields (). The reactions were completed in 30–70 min with excellent yields. The reaction for the aldehydes (c) and (o) proceeded fast in comparison with other substrates. The aldehydes with electron-deficient substituents (entry b) gave poor yields while on the contrary aldehydes with electron-rich substituents (entry c) gave good yields. Even a bulky molecule like aldehyde (p), which is an intermediate used in synthesis of the antiobesity drug fluvastatin, underwent the reaction with a good yield.
Table 2. Oxathioacetalization of aldehydes.
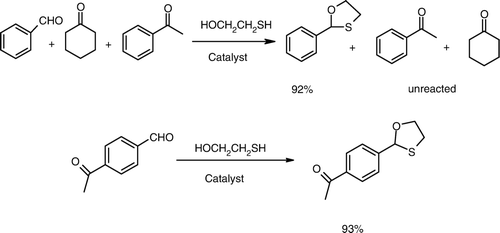
Taking into account the mildness of the catalyst, we decided to study the chemoselectivity. For that we took equimolar amounts (2mmole each) of cyclohexanone, acetophenone, benzaldehyde, and mercaptoethanol and stirred the reaction mixture at room temperature with the catalyst (50mg). After 2 h of reaction, only aldehyde underwent the reaction and (from GC analysis) the ketones remained unreacted (Scheme 1). This kind of selectivity is not found even with the silica supported polyphosphoric acid (PPA.SiO2) Citation18, making it clear that H3PO4. SiO2 is milder than this catalyst. In the case of 4-acetyl benzaldehyde the chemoselective oxathioacetalization (93% conversion) of aldehyde was observed. We have compared this catalyst with some recently reported catalysts. The present catalyst is simple owing to its easy preparation and workup procedures and good yields under solvent-free conditions ().
Table 3. Comparison of some recent results with present protocol.
Reusability of the catalyst
To test the reusability of the catalyst, benzaldehyde was treated with mercaptoethanol in the presence of the catalyst. After completion of the reaction (TLC), ethyl acetate was added and filtered to get the solid catalyst. This, after vacuum drying at 80°C, was reused for the reaction. The catalyst was reused without any substantial loss of catalytic activity for four consecutive cycles ().
Table 4. Reusability of the catalyst.
Experimental
Silica gel (100–200 mesh, Aldrich), orthophosphoric acid (85% aqueous, Aldrich), chloroform (AR grade, Runa chemicals, India), and ethyl acetate (AR grade, Runa chemicals, India) were used as received. The mercaptoethanol (caution: avoid inhalation and contact with skin) and dihydropyran were purchased from Aldrich and were used as received. The aldehydes were either purchased from Aldrich or prepared in the lab and were used as is. The progress of the reaction was monitored by TLC (Silica gel 60 F254). Mass spectra were obtained using GC/MS Shimadzu QP-2010 (EI, 70eV). The samples for SEM were prepared in an auto fine sputter coater with a gold target. The SEM-EDX characterization was performed on a JEOL JSM-6360 scanning electron microscope equipped with a JEOL EX-54175JMU energy dispersive X-ray high vacuum germanium detector (E= 20 kV, W-filament). Samples were analyzed from 15 mm working distance, with a resolution of 12 nm. The content of phosphorous was also measured by EDX. IR spectra were recorded on a Perkin Elmer Spectrometer. The 1H NMR and 13C NMR were recorded on a JEOL 300 MHz/ Bruker 300 MHz Avance II spectrometer in CDCl3.
Preparation of silica supported orthophosphoric acid catalyst
Orthophosphoric acid (3.5 gm) was added to a suspension of SiO2 (5 g) in chloroform (30 mL) and stirred at room temperature for 2h. The mixture was concentrated and heated at 100°C to get free-flowing powder.
Oxathioacetalization of aldehydes
A stirred mixture of aldehyde (2mmol) and 2-mercaptoethanol (2.4mmol, 0.17mL) was treated with the catalyst (50mg) at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction (TLC), the reaction mixture was diluted with ethyl acetate and the catalyst was filtered off. The organic layer was washed with bicarbonate solution, then with water, and dried over anhydrous sodium sulfate. Then the filtrate was concentrated and purified by silica gel column chromatography.
Conclusions
A simple and recyclable silica supported orthophosphoric acid catalyst has been prepared, characterized, and used to devise a mild and efficient protocol for chemoselective oxathioacetalization of aldehydes. The yields are excellent with chemoselective reaction of the aldehydes. The catalyst can be called green because of its ease of preparation, mildness, easy workup procedure (filtration), and stability in air. Apart from this, the solvent-free reaction conditions and applicability to a wide spectrum of aldehydes are other advantages of the present protocol.
Acknowledgements
ADS, DGR, and ARD are thankful to DRDO and DST New Delhi for their financial support and to Dr P.P. Wadgaonkar and Dr C.V. Rode, NCL, Pune, India, for fruitful discussions.
References
- Deshmukh , A.P. ; Padiya , K.J. ; Salunkhe , M.M. J. Chem. Res. (S) 1999 , 568 – 569 .
- Dondoni , A. ; Massi , A. Tetrahedron Lett . 2001 , 42 , 7975 – 7978 .
- Gonzalez-Nunez, M.E.; Mello , R. ; Olmos , A. ; Asensio , G. J. Org. Chem . 2005 , 70 , 10879 – 10882 .
- Clark , J.H. ; Macquarrie , D.J. ; Mubofu , E.B. Green Chem . 2000 , 2 , 53 – 55 .
- Kamitori , Y. ; Hojo , M. ; Masuda , R. ; Kimura , T. ; Yoshida , T. J. Org. Chem . 1986 , 51 , 1427 – 1431 .
- Patney , H.K. Tetrahedron Lett . 1994 , 35 , 5717 – 5718 .
- Patney , H.K. ; Margan , S. Tetrahedron Lett . 1996 , 37 , 4621 – 2622 .
- Chandrasekhar , S. ; Takhi , M. ; Ravindra , Y.R. ; Mohapatra , S. ; Rama , C.R. ; Venkatraman , K.R. Tetrahedron 1997 , 53 , 14997 – 15004 .
- Vijaya , R.A. ; Saravanan , P. ; Singh , V.K. Synlett 1999 , 4 , 415 – 416 .
- Khan , A.T. ; Pravin , T. ; Choudhury , L.H. Synthesis 2006 , 15 , 2497 – 2502 .
- Aoyama , T. ; Okada , K. ; Okada , H. ; Nakajima , H. ; Matsumoto , T. ; Takido , T. ; Kodumari , M. Synlett 2007 , 3 , 387 – 390 .
- Chakraborti , A.K. ; Gulhane , R. Chem. Commun . 2003 , 46 ( 15 ), 1896 – 1897 .
- Du , Y. ; Wei , G. ; Cheng , S. ; Hua , Y. ; Linhardt , R.J. Tetrahedron Lett . 2006 , 47 , 307 – 310 .
- Narasimhulu , M. ; Srikanth Reddy , T. ; Chinni Mahesh , K. ; Prabhakar , P. ; Bhujanga Rao , C. ; Venkateswarlu , Y. J. Mol. Catal. A: Chem . 2007 , 266 , 114 – 117 .
- Fey , T. ; Fischer , H. ; Bachmann , S. ; Abert , K. ; Bolm , C. J. Org. Chem . 2001 , 66 , 8154 – 8159 .
- Dias , A.P.S. ; Rozanov , V.V. ; Waerenborgh , J.C.B. ; Portela , M.F. Appl. Catal., A 2008 , 345 , 185 – 194 .
- Chakraborti , A.K. ; Singh , B. ; Chankeshwara , S.V. ; Patel , A.R. J. Org. Chem . 2009 , 74 ( 16 ), 5967 – 5974 .
- Aoyama , T. ; Takido , T. ; Kodomari , M. Synlett 2004 , 13 , 2307 – 2310 .
- Kamble , V.T. ; Bandgar , B.P. ; Muley , D.B. ; Joshi , N.S. J. Mol. Catal. A: Chem . 2007 , 268 , 70 – 75 .
- Khan , A.T. ; Sahu , P.R. ; Majee , A. J. Mol. Catal. A: Chem . 2005 , 226 , 207 – 212 .
- Srivastava , N. ; Dasgupta , S.K. ; Banik , B.K. Tetrahedron Lett . 2003 , 44 , 1191 – 1193 .
- Ali , M.H. ; Gomes , M.G. Synthesis 2005 , 1326 – 1332 .
- Kumar , A. ; Kumar , S. ; Saxena , A. ; De , A. ; Mozumdar , S. Catal. Lett . 2008 , 122 ( 1–2 ), 98 – 105 .
- Liang , X. ; Gao , S. ; Yang , J. ; He , M. Catal. Commun . 2008 , 10 , 156 – 159 .
- Hazarika , P. ; Sharma , S.D. ; Konwar , D. Catal. Commun . 2008 , 9 , 2398 – 2402 .
- Firouzabadi , H. ; Iranpoor , N. ; Karimi , B. Synthesis 1999 , 1 , 58 – 60 .
- De , S.K. Synthesis 2004 , 828 – 830 .
- Chandrasekhar , S. ; Jaya Prakash , S. ; Shyamsunder , T. ; Ramchander , T. Synth. Commun . 2005 , 35 , 3127 – 3131 .
- Li , Y. ; Zhang , X. ; Ren , T. ; Zhou , J. Synth. Commun . 2006 , 36 , 1679 – 1685 .