Abstract
Polyethylene glycol (PEG) is found to be an inexpensive, non-toxic, environmentally friendly reaction media for the synthesis of pyrrolo[2,3-d] isoxazoles by using NaOCl reagent in excellent yields under mild conditions.
 1
1
Introduction
In performing the majority of organic transformations, solvents play an important role in homogeneously mixing the system and allowing molecular interactions to be more efficient. But the toxic and volatile nature of many organic solvents that are widely used in organic synthesis have posed a serious threat to the environment. To address some of the issues, green chemistry approach holds out significant potential not only for reduction of by-products, a reduction in the waste produced, lowering of energy costs, and development of new methodologies Citation1. More recently attention has been drawn to the development of environmentally benign solvents Citation2 . In fact, the value of a new solvent system primarily depends on its environmental impact, the ease with which it can be disposed, the number of times the solvent can be recycled, low vapor pressure, non-flammability and high polarity for solubilization.
In this article, we describe the use of a simple and widely available polymer polyethylene glycol (PEG) as alternative green reaction media. With unique properties such as thermal stability, commercial availability, non-volatility, immiscibility with a number of organic solvents, and recyclability, PEG is a biologically acceptable polymer used extensively in drug delivery and in bio-conjugates as a tool for diagnostics, and has now been used as an environmental friendly solvent medium Citation3.
Pyrroles are important heterocycles broadly used in material science Citation4 and are found in naturally occurring and biologically important molecules. Pyrroles can be found in a tremendous range of natural products Citation5 and bioactive molecules Citation6. Within these, pyrrole fused heteroaromatic molecules are scarce in nature, but a significant number of compounds have been synthesized in laboratories.
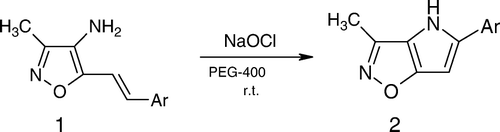
Looking for a valuable procedure for the synthesis of pyrrolo[2,3-d]isoxazoles, several approaches have been investigated. One method is by de-oxygenative cyclization of nitrostyrylisoxazoles using triethylphosphite (TEP) Citation7–9. As the reaction requires high temperature, this results in cleavage of the isoxazole ring, which is unstable at high temperature. To overcome this problem, another method developed by Carotti Citation10 is employed, which involves the reductive cyclization of nitrostyrylisoxazoles using SnCl2 in N, N-dimethylformamide (DMF). However, we could not achieve the synthesis of the target compounds using this procedure. Soderberg et al. Citation11 did not achieve the reductive N-heterocyclization of nitrostyrylisoxazoles by palladium-catalyzed carbon monoxide-mediated method, even though they could able to synthesize a variety of fused pyrroles. We decided to construct pyrrol-[2,3-d]isoxazoles by utilizing aminostyrylisoxazoles rather than nitrostyrylisoxazoles by Sharpless epoxidation Citation12 . However, this method requires high reaction times, specific reagents and catalysts, which are not environmentally benign, and is costlier. More recently, we were successful in constructing the title compounds by reductive cyclization of nitrostyrylisoxazoles by using SnCl2.2H2O in ionic liquids Citation13 . But ionic liquids require tedious preparation procedure and their environment safety is still debatable moreover SnCl2 is toxic and not recommended as a reagent from the green chemistry point of view. Continuing our efforts in sustainable synthesis development, with reference to isoxazoles Citation14–18, we describe a rapid and efficient one pot synthesis of title compounds by using environmentally friendly reagent NaOCl as well as green reaction media PEG from aminostyrylisoxazoles ().
Results and discussion
To establish suitable experimental conditions for the reaction, we initially reacted 4-amino-3-methyl-5-styrylisoxazoles Citation1 Citation19, with NaOCl in various reaction media like chloroform, THF, acetonitrile and water. The reaction has not proceeded. However, we obtained the desired product Citation2 by using THF/H2O and CH3CN/H2O, but the yields are consistently low and requires high reaction times even though on addition of excess quantity of NaOCl with extended reaction times. ( , entry 1, 2). Subsequently, we examined the reaction in PEG-400 medium. To our delight, reaction was completed in 1.5 h, with enhanced yields. This may be due to the attraction between the PEG hydroxyl group oxygen and hydrogen attached to nitrogen of amine. This makes the N–H bond weaker, enhancing the nucleophilicity of nitrogen.
Table 1. Optimization of reaction conditions.
Encouraged by these results, we further examined the scope of our methodology with a variety of aminostyrylisoxazoles, and the results are presented in . Finally, the results indicate that our method is compatible with various functional groups and the approach proved to be of general applicability. All the synthesized compounds are known and identified by spectroscopic data, and identical with reported physical properties Citation12 . This synthetic strategy permits the introduction of a diverse array of substituents on to the benzene ring. To the best of our knowledge, this report is the first of its kind to construct a pyrrole ring employing NaOCl reagent in PEG media.
Table 2. Synthesis of 3-methyl-5-aryl-4H-pyrrolo[2,3-d]isoxazoles in PEG.
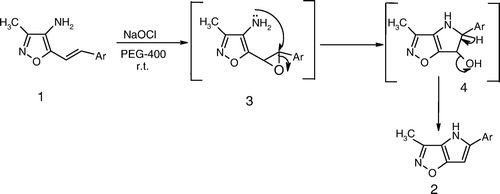
As shown in , these reactions also give the expected products in good yields. The proposed mechanism is illustrated in .
The reaction mechanism may initially involve the formation of an epoxide Citation3 by epoxidation of styryl double bond, which is subsequently ring-opened by nucleophilic attack of amino functional group to give the cyclic intermediate Citation4. This could undergo dehydration to give the product Citation2. Even though, we could not isolate the epoxide Citation3 in this case, the evidence for the formation of an epoxide during the reaction has been established by comparing the TLC of an epoxide which was reported by us earlier Citation12, with the epoxide produced in this reaction by monitoring the reaction with TLC from time to time (identical Rf values). This gives ample evidence for the formation of epoxide during the course of the reaction.
Experimental
PEG-400 was purchased from Aldrich. All the melting points were determined on a Cintex melting point apparatus and are uncorrected. Analytical TLC was performed on Merck precoated 60 F254 silica gel plates. Visualization was done by exposing to iodine vapor. Column chromatography was conducted by using silica gel with ethylacetate – n-hexane solvent system as elute. IR spectra (KBr pellet) were recorded on a Perkin-Elmer BX series FT-IR spectrometer. 1H NMR spectra were recorded on a Varian Gemini 300 MHz spectrometer. 13C NMR spectra were recorded on a Bruker 75 MHz spectrometer. Chemical shift values are given in ppm (δ) with tetramethyl silane as internal standard. Mass Spectral measurements were carried out by EI method on a Jeol JMC-300 spectrometer at 70 ev. Elemental analyses were performed on a Carlo Erba 106 and Perkin-Elmer model 240 analyzers.
General procedure for the synthesis of pyrrol[2,3-d]isoxazoles
To aminostyrylisoxazole (1 mmol) in PEG-400 (5 mL), NaOCl (2 mmol) was added and stirred at room temperature. On completion of the reaction, as was indicated by TLC, ether (10 mL) was added, the mixture stirred for 2 min and reaction mixture was allowed to settle for 5 min. Cooling in an acetone dry ice-bath caused solidification of solvent medium. This allowed us to decant the ether layer. The sequence was repeated twice with 10 mL portion of ether the combined ether layers were concentrated under reduced pressure and the resulting crude product was purified by silica gel column chromatography using ethyl acetate and hexane as an eluent to obtain the product in excellent yield. The recovered solvent medium can be reused after liquefication by bringing to room temperature for further runs (). All the products are fully characterized by spectral analysis.
Table 3. Recycling of PEG.
Spectral data for selected compounds
3-Methyl-5-aryl-4H-pyrrolo[2,3-d]isoxazole 2a
Yield (94%), mp 155–156°C; IR (KBr) cm–1 3255, 1600, 980; 1H NMR (CDCl3, 300 MHz). δ 2.50 (s, 3H), 7.30–7.60 (m, 5H), 7.75 (s, 1H), 7.90 (s, 1H, NH, D2O exchangeable); 13C NMR (CDCl3, 75 MHz): δ 12.8, 100.5, 110.4, 128.1, 129.5, 131.2, 133.0, 136.3, 143.3, 152.5. MS (EI): m/z 198 [M+].
3-Methyl-5-(4-methyl)aryl-4H-pyrrolo[2,3-d]isoxazole 2b
Yield (92%), mp 145–151°C; IR (KBr) cm–1 3350, 1615, and 975; 1H NMR (CDCl3, 300 MHz): 0 2.40 (s, 3H), 2.82 (s, 3H), 7.31–7.50 (m, 4H), 7.85 (s, 1H), 7.96 (s, 1H, NH, D2O exchangeable); 13C NMR (CDCl3 75 MHz): δ 11.5, 22.1, 100.5, 112.6, 127.8, 128.5, 131.0, 133.5, 137.5, 145.0, 158.0. MS (EI): m/z 212 [M+].
Conclusions
We have developed an efficient protocol for the construction of pyrrolo- [2,3-d]isoxazoles in PEG as the green solvent by using environmentally benign reagent NaOCl. PEG not only serves as a reaction medium, but also significantly enhances the reactivity. The advantages of this methodology are a easy operation, b mild reaction conditions,c recyclability of the solvent. These advantages make this process potentially useful for industrial application.
Acknowledgements
The authors are thankful to the Head, department of Chemistry, Kakatiya University, Warangal, India, for providing the facilities. K. Govardhan Reddy is thankful to UGC, New Delhi, for the award of a fellowship (JRF).
References
- Kumar , G.D.K. ; Baskaran , S. Chem. Commum . 2004 , 8 , 1026 – 1027 .
- Meshram , H.M. ; Reddy , P.N. ; Vishnu , P. ; Sadashiv , K. ; Yadav , J.S. Tetrahedron Lett . 2006 , 47 , 991 – 995 .
- Chandrasekhar , S. Narsihmulu , Ch. ; Chandrashekar , G. ; Shyamsunder , T. Tetrahedron Lett . 2004 , 45 , 2421 – 2423 .
- Ogawa , K. ; Rasmussen , R.C. J. Org. Chem . 2003 , 68 , 2921 – 2928 .
- Banwell , M.G. ; Smith , J.A. J. Chem., Perkin Trans 1 . 2002 , 23 , 2613 – 2618 .
- Muchowski , J.M. Adv. Med. Chem . 1992 , 1 , 109 – 135 .
- Sudberg , R. J. Org. Chem . 1965 , 30 , 3604 – 3610 .
- Rao , C.P. ; Rao , V.K. , Sundaramurthy , V. Synthesis . 1981 , 3 , 234 – 236 .
- Mali , R.S. ; Yadav , V.J. Synthesis . 1984 , 10 , 862 – 865 .
- Stefanachi , A. ; Leonetti , F. ; Cappa , A. ; Carotti , A. Tetrahedron Lett . 2003 , 44 , 2121 – 2123 .
- Gorugantula , S.P. ; Martinez , G.M.C. ; Dantale , S.W. ; Soderberg , C.G. Tetrahedron . 2010 , 66 , 1800 – 1805 .
- Rajanarendar , E. ; Mohan , G. ; Ramesh , P. ; Karunakar , D. Tetrahedron Lett . 2006 , 47 , 4957 – 4959 .
- Rajanarendar , E. ; Raju , S. ; Reddy , A.S.R. ; Reddy , K.G. ; Reddy , M.N. Chem. Pharm. Bull . 2010 , 58 , 833 – 839 .
- Rajanarendar , E. ; Ramesh , P. ; Rao , E.K. ; Mohan , G. ; Srinivas , M. Arkivoc . 2007 , xiv , 266 – 275 .
- Rajanarendar , E. ; Mohan , G. ; Ramesh , P. ; Srinivas , M. J. Hetrocyclic Chem . 2007 , 44 , 215 – 217 .
- Rajanarendar , E. ; Ramesh , P. ; Srinivas , M. ; Ramu , K. ; Mohan , G. Synth. Commun . 2006 , 36 , 665 – 671 .
- Rajanarendar , E. ; Rao , E.K. ; Shaik , F.P. ; Reddy , M.N. ; Srinivas , M. J. Sulfur Chem . 2010 , 31 , 263 – 274 .
- Rajanarendar , E. ; Reddy , M.N. ; Murthy , K.R. ; Reddy , K.G. ; Raju , S. ; Srinivas , M. ; Praveen , B. ; Rao , M.S. Bioorg. Med. Chem. Lett . 2010 , 20 , 6052 – 6055 .
- Murthy , A.K. ; Rao , K.S.R.K.M. J. Indian Chem. Soc . 1976 , 53 , 1047 – 1048 .