Abstract
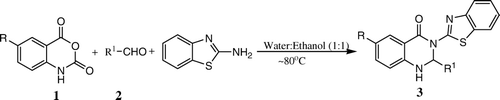
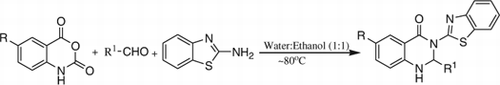
An efficient and environmentally benign protocol for the synthesis of 3-(2′-benzothiazolyl)-2,3-dihydroquinazolin-4(1H)-ones has been developed. The synthesis of these pharmacologically important compounds can be achieved by the three component condensation of isatoic/5-chloroisatoic anhydride, aldehyde, and 2-aminobenzothiazole in water: ethanol (1:1, v/v) without the aid of any catalyst.
Introduction
The art of performing multicomponent reactions in a one-pot operation has received considerable attention due to their ability to generate molecular diversity and complexity Citation1–6. These are, therefore, ideally suited for generating libraries of small molecules and particularly heterocyclic compounds Citation7 Citation8. 2,3-Dihydroquinazolin-4(1H)-ones have emerged as versatile biologically active compounds spanning applications as antitumor, diuretic, herbicides agents, and plant growth regulators Citation9–12. The classical synthesis of 2,3-dihydroquinazolin-4(1H)-ones involves the condensation reaction of anthranilamide with aldehyde or ketone in presence of p-toluenesulfonic acid under vigorous conditions Citation13 Citation14. Some of the other methods reported for the synthesis of these derivatives involve condensation of o-aminobenzamides with benzil followed by base hydrolysis Citation15, reductive cyclization of o-nitrobenzamides Citation16–20, and so on. Our group has reported a new method for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones by reductive desulfurization of 2-thioxo-3H-quinazolin-4-ones with nickel boride at ambient temperature Citation21. Some recent methods include one pot-pot three-component condensation of aldehydes, isatoic anhydride, and primary amines or ammonium acetate Citation22–24. Some of the procedures suffer from certain drawbacks such as expensive catalysts, harsh reaction conditions, or low yields. Shaabani et al. have also reported these synthesis in ionic liquids under neat conditions Citation25 Citation26 in good yields.
The use of environmentally benign solvents such as water and solvent free neat reactions represent powerful green procedures economically and environmentally. Water is one of the most abundant, cheapest, and environmentally friendly solvents and it also exhibits unique reactivity and selectivity different from conventional organic solvents Citation27–31. In view of the above, we have investigated the synthesis of 3-(2′-benzothiazolyl)-2,3-dihydroquinazolin-4(1H)-ones under variety of conditions by a one-pot three component condensation of isatoic/5-chloroisatoic anhydride, aldehyde and 2-aminobenzothiazole in aqueous medium with and without any co-solvents but without the aid of any catalyst.
Results and discussion
In this paper, we report a green synthesis of benzothiazoquinazolinone derivatives by one-pot three component condensation of 2-aminobenzothiazole, aldehydes, and isatoic/5-chloroisatoic anhydride in water-ethanol (1:1, v/v) without any catalyst. The reactions in water were very slow. The reaction conditions were optimized by carrying out the reaction of isatoic anhydride (1 mmol), benzaldehyde (1 mmol), and 2-aminobenzothiazole (1 mmol) in different solvents. Initially, a reaction was attempted using acetonitrile as solvent. There was no reaction at all at room temperature. The contents were then heated at ~80°C. The reaction was found to be incomplete after 5 h but showed the formation of a new product. The product was separated and characterized by NMR, IR, and mass spectra to be 3-(2′-benzothiazolyl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3a) in 25% yield. The reaction was then attempted in other solvents such as dichloromethane, toluene, methanol, ethanol, and water. The reactions were incomplete even after 5 h. However, reactions in ethanol and water, unlike other solvents, gave reasonably high yields of the product, 70 and 62%, respectively. Therefore, a reaction was attempted in water-ethanol mixture (1:1, v/v) at ~80°C without any catalyst. The reaction was complete in 20 min and 92% of the product 3a was obtained after a simple work up. A comparative survey for the use of different solvents is drawn in .
Table 1. Effect of solvent on condensation reaction of isatoic anhydride, benzaldehyde, and 2-aminobenzothiazole.
Thus, ethanol-water (1:1, v/v) mixture was found to be the best solvent system for the condensation. After having optimized the reaction conditions, the generality of the reaction was confirmed by carrying out the reactions of several other aldehydes with 2-aminobenzothiazole and isatoic anhydride or 5-chloroisatoic anhydride in water-ethanol (1:1, v/v) at ~80°C. A series aromatic aldehydes containing electron withdrawing and electron releasing groups, aliphatic and heteroaromatic aldehydes underwent successful multi-component condensation in short reaction times to give high yields of the corresponding 3-(2′-benzothiazolyl)-2,3-dihydroquinazolin-4(1H)-ones ().
The products were characterized by IR, 1H-NMR, 13C-NMR, and mass spectral studies. IR spectra showed prominent sharp N–H stretching in region 3350–3370. The 1H-NMR spectra showed two doublets, one for methine proton and other for N–H proton in region δ 7.5–8.5. The 13C-NMR showed characteristic peak of C-sp3 in region δ 65–70. The results are presented in . Though the compounds 3a-f are known, a number of new compounds 3g-t have been synthesized by this methodology.
Table 2. One pot synthesis of 3-(2′-benzothiazolyl)-2,3-dihydroquinazolin-4(1H)-ones in ethanol:water (1:1) at ~80°C.
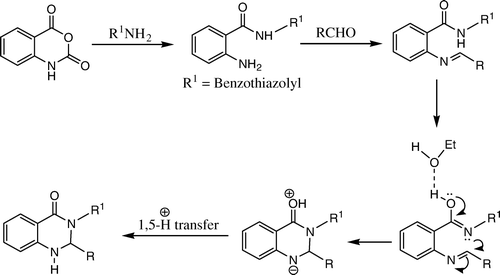
The probable pathway for these condensations is given in . In summary, we have described an easy, efficient, and green protocol for the synthesis of 3-(2′-benzothiazolyl)-2,3-dihydroquinazolin-4(1H)-one in aqueous medium. The procedure offers several significant advantages such as operational simplicity, mild reaction conditions, easy isolation of products, good yields, and low reaction times.
Experimental
Melting points were recorded on a Tropical Labequip apparatus and are uncorrected. The products were confirmed by their mp, IR, NMR, and mass spectra. IR spectra were recorded on Perkin-Elmer FTIR-1710 instrument. 1H and 13C spectra were recorded on Bruker Avance Spectrospin 300 MHz using TMS as internal standard. Mass spectra were recorded on KC-455-TOF mass spectrometer (Micromass, Manchester, UK).
General procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones
In a typical experiment, a 100 mL round-bottomed flask fitted with a reflux condenser was mounted on a magnetic stirrer. A mixture of isatoic anhydride (5 mmol, 0.81 g), benzaldehyde (5 mmol, 0.53 g), and 2-aminobenzothiazole (5 mmol, 0.75 g) was placed into it. The 20 mL of water-ethanol mixture (1:1, v/v) was added to the flask and the mixture was heated with stirring in an oil-bath maintained at ~80°C for the appropriate time as mentioned in . After the complete disappearance of the starting material, as monitored by TLC (eluent: petroleum ether: ethyl acetate: 80:20), the reaction mixture was cooled to room temperature. The solid product obtained was filtered at pump, washed with water-ethanol, and dried to obtain 2.48 g (92%) of the crude product. The crude product was recrystallized from ethanol to yield pure 3-(2′-benzothiazolyl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one.
Selected spectral data
3-(2′-Benzothiazolyl)-2,3-dihydro-2-(4-fluorophenyl)-quinazolin-4(1H)-one (3g)
Creamish solid; yield = 82%; M.p. 252–254°C; IR (KBr) νmax/cm–1: 3374, 1640, 1616, 1510, 1435, 1236, 745; 1H-NMR (300 MHz, DMSO): δ 6.81 (t, J=7.5 Hz, 1H), 6.95 (d, J=8.1 Hz, 1H), 7.13 (t, J=8.6 Hz, 2H), 7.30–7.48 (m, 5H, Ar), 7.54 (d, J=3.3 Hz, 1H, CH (sp3), 7.77–7.81 (m, 2H, Ar), 8.05 (d, J=7.7 Hz, 1H), 8.30 (d, J=3.5 Hz, 1H, NH); 13C-NMR (300 MHz, DMSO): δ 67.47, 113.52, 115.32, 118.54, 120.94, 121.76, 124.15, 126.30, 127.83, 127.94, 128.41, 135.58, 135.75, 146.70, 147.60, 147.60, 157.65, 160.16, 161.47, 163.41; MS (ESI) m/z 376.3743 (M + H)+.
3-(2′-Benzothiazolyl)-2,3-dihydro-2-(4-dimethylaminophenyl)-quinazolin-4(1H)-one (3i)
Creamish solid; yield = 85%; M.p. 212–214°C; IR (KBr) νmax/cm–1: 3356, 1639, 1617, 1513, 1159, 750; 1H-NMR (300 MHz, DMSO): δ 2.79 (s, 6H, 2×CH3), 6.57 (d, J=8.7 Hz, 2H), 6.76 (t, J=7.4 Hz, 1H), 6.89 (d, J=8.1 Hz, 1H), 7.10 (d, J=8.7 Hz, 2H), 7.30–7.43 (m, 3H, Ar), 7.44 (d, J=3.4 Hz, 1H, CH (sp3), 7.77 (t, J=8.7 Hz, 2H), 7.98 (d, J = 7.7 Hz, 1H), 8.13 (d, J=3.6 Hz, 1H, NH); 13C-NMR (300 MHz, DMSO): δ 33.60, 68.48, 111.60, 114.65, 115.68, 118.51, 120.96, 121.07, 123.65, 125.77, 126.95, 128.77, 131.15, 132.00, 133.16, 134.92, 146.77, 148.17, 150.13, 158.04, 162.19; MS (ESI) m/z 401.7945 (M + H)+.
3-(2′-Benzothiazolyl)-2,3-dihydro-2-isopropyl-quinazolin-4(1H)-one (3k)
Off white solid; yield = 82%; M.p. 194–195°C; IR (KBr) νmax/cm–1: 3362, 1636, 1620, 1513, 1270, 750; 1H-NMR (300 MHz, DMSO): δ 0.73 (d, J=6.7 3H, CH3), 0.85 (d, J=6.7 Hz, 3H, CH3), 2.19 (m, 1H, >CH), 6.15 (m, 1H, CH), 6.72 (t, J=7.4 Hz, 1H), 6.84 (d, J=8.2 Hz, 1H), 7.24–741 (m, 3H, Ar), 7.70 (d, J=3.9 Hz, 1H (sp3), 7.74 (d, J=7.9 Hz, 2H), 7.92 (d, J = 4.8 Hz, 1H); 13C-NMR (300 MHz, DMSO): δ 17.92, 18.13, 32.04, 71.94, 113.56, 114.74, 117.31, 120.45, 123.14, 125.33, 128.08, 132.51, 134.45, 146.58, 147.57, 158.01, 161.41; MS (ESI) m/z 323.5962 (M+).
3-(2′-Benzothiazolyl)-2,3-dihydro-2-(2-pyridyl)-quinazolin-4(1H)-one (3m)
Creamish solid; yield = 83%; M.p. 228–230°C; IR (KBr) νmax/cm–1: 3352, 1638, 1614, 1436, 1239, 747; 1H-NMR (300 MHz, DMSO): δ 6.77 (t, J=7.4 Hz, 1H), 6.87 (d, J = 8.1 Hz, 1H), 7.24 (t, J=5.2 Hz, 1H), 7.30–7.55 (m, 5H, Ar), 7.70–7.81 (m, 3H, Ar), 8.0 (d, J=7.7 Hz, 1H), 8.24 (d, J=3.8 Hz, 1H, CH (sp3), 8.35 (d, J=4.3 Hz, 1H, NH); 13C-NMR (300 MHz, DMSO): δ 68.84, 113.98, 115.53, 118.36, 120.78, 120.89, 121.62, 123.50, 123.90, 126.12, 128.25, 132.62, 135.10, 137.20, 146.45, 147.63, 148.72, 157.52, 158.01, 161.89; MS (ESI) m/z 358.2618 (M+).
3-(2′-Benzothiazolyl)-6-chloro-2,3-dihydro-2-(4-isopropylphenyl)-quinazolin-4(1H)-one (3r)
Creamish solid; yield = 91%; M.p. 235–237°C; IR (KBr) νmax/cm–1: 3362, 1635, 1621, 1507, 1426, 1269, 1221, 814, 758; 1H-NMR (300 MHz, DMSO): δ 1.08 (d, J=6.9 Hz, 6H, 2xCH3), 2.51–2.80 (m, 1H, >CH), 6.99 (d, J=9.0 Hz, 1H), 7.14–7.22 (m, 4H), 7.33–7.47 (m, 3H), 7.56 (d, J=3.6 Hz, 1H, CH (sp3), 7.77 (t, J=9.3 Hz, 2H), 8.05 (d, J=7.5 Hz, 1H), 8.52 (d, J=3.9 Hz, 1H); 13C-NMR (300 MHz, DMSO): δ 23.55, 23.59, 32.94, 67.89, 114.45, 117.87, 120.99, 121.76, 121.97, 124.22, 125.67, 126.32, 126.57, 127.20, 132.58, 135.24, 136.57, 145.68, 147.57, 148.58, 157.49, 160.62; MS (ESI) m/z 433.8831 (M+), 435.8846 (M++2).
3-(2′-Benzothiazolyl)-6-chloro-2,3-dihydro-2-hexyl-quinazolin-4(1H)-one (3s)
Light yellow solid; yield = 81%; M.p. 222–224°C; IR (KBr) νmax/cm–1: 3360, 1638, 1516, 1428, 1229, 757; 1H-NMR (300 MHz, DMSO): δ .80 (t, J=6.3 Hz, 3H), 1.19–1.33 (m, 8H), 1.66–1.91 (m, 2H), 6.38–6.43 (m, 1H), 6.95 (d, J=8.7 Hz, 1H), 7.35 (t, J=7.5 Hz, 1H), 7.43–750 (m, 2H), 7.76 (d, J=2.4 Hz, 1H, CH (sp3), 7.82 (d, J=8.1 Hz, 1H), 7.88 (d, J=3.6 Hz, 1H, NH), 8.01 (d, J=7.5 Hz, 1H); 13C-NMR (300 MHz, DMSO): δ 13.86, 21.94, 24.42, 27.87, 30.97, 32.03, 67.62, 113.99, 117.68, 120.90, 121.49, 121.63, 124.04, 126.22, 127.21, 132.48, 135.1, 145.49, 147.66, 157.02, 160.04; MS (ESI) m/z 399.5097 (M+), 401.5144 (M++2).
Acknowledgements
SK thanks CSIR New Delhi (India) for the grants of junior and senior research fellowships.
References
- Kappe , C.O. Curr. Opin. Chem. Biol . 2002 , 6 , 314 – 320 .
- Domling , A. ; Ugi , I. Angew. Chem. Int. Ed . 2000 , 39 , 3168 – 3210 .
- Zhu , J. ; Bienayme H. Multicomponent Reactions ; Weinheim : Wiley-VCH , 2005 .
- Pandey , G. ; Singh , R.P. ; Singh , V.K. Tetrahedron Lett . 2005 , 46 , 2137 – 2140 .
- Kappe , C.O. Acc. Chem. Res . 2000 , 33 , 879 – 888 .
- Terret , N.K. ; Gardner , M. ; Gordon , D.W. ; Kobylecki , R.J. Steele , J. Tetrahedron 1995 , 51 , 8135 – 8173 .
- Weber , L. ; Illgen , K. ; Almstetter , M. Synlett. 1999 , 366 – 374 .
- Bienayme , H. ; Hulme , C. ; Oddon , G. ; Schmitt , P. Chem. Eur. J. 2000 , 6 , 3321 – 3329 .
- Biressi , M.G. ; Cantarelli , G. ; Carissimi , M. ; Cattaneo A. ; Ravenna , F. Farmaco Ed. Sci . 1969 , 24 , 199 – 220 .
- Bhalla , P.R. ; Walworth , B.L. American Cyanamid Co., Eur. Pat. Appl. EP 58, 822 , Chem. Abstr . 1983 , 98 , 1669 ; Bhalla, P.R.; Walworth, B.L. American Cyanamid Co., US Patent 4,431,440, Chem. Abstr. 1984, 100, 174857 .
- Hamel , E. ; Lin , C.M. ; Plowman , J. ; Wang , H. ; Lee K. ; Paull , K.D. Biochem. Pharmacol . 1996 , 51 , 53 – 59 .
- Hour , M. ; Huang , L. ; Kuo , S. ; Xia , Y. ; Bastow , K. ; Nakanishi , Y. ; Hamel E. ; Lee , K. J. Med. Chem . 2000 , 43 , 4479 – 4487 .
- Sharma , S.D. ; Kaur , V. Synthesis 1989 , 677 – 680
- Su , W.K. ; Yang , B.B. Aust. J. Chem . 2002 , 55 , 695 – 697 ; Su, W.K.; Yang, B.B. J. Chem. Res., Synop. 2002, 604–605 .
- Moore , J.A. ; Sutherland , G.J. ; Sowerby , R. ; Kelly , E.G. ; Palermo , S. ; Webster , W. J. Org. Chem . 1969 , 64 887 – 892 .
- Shi , D.Q. ; Rong , L.C. ; Wang , J.X. ; Wang , X.S. ; Tu , S.J. ; Hu , H.W. Chem. J. Chin. Univ . 2004 , 25 , 2051 – 2054 .
- Shi , D.Q. ; Wang , J.X. ; Rong , L.C. ; Zhuang , Q.Y. ; Tu , S.J. ; Hu , H.W. J. Chem. Res. Synop . 2003 , 671 – 673 .
- Shi , D.Q. ; Rong , L.C. ; Wang , J.X. ; Zhuang , Q.Y. ; Wang , X.S. ; Hu , H.W. Tetrahedron Lett . 2003 , 44 , 3199 – 3201 .
- Shi , D.Q. ; Shi , C.L. ; Wang , J.X. ; Rong , L.C. ; Zhuang , Q.Y. ; Wang , X.S. J. Heterocyclic Chem . 2005 , 40 , 173 – 183 .
- Cai , G.P. ; Xu , X.L. ; Li , Z.F. ; Weber , W.P. ; Lu , P. J. Heterocyclic Chem . 2002 , 39 , 1271 – 1272 .
- Khurana , J.M. ; Kukeraja , G. J. Heterocyclic Chem . 2003 , 40 , 677 – 679 .
- Chen , J. ; Wu , D. ; He , F. ; Liu , M. ; Wu , H. ; Ding , J. ; Su , W. Tetrahedron Lett . 2008 , 49 , 3814 – 3818 .
- Rostamizadeh , S. ; Amani , A.M. ; Aryan , R. ; Ghaieni , H.R. ; Shadjou , N. Synth. Commun. 2008 , 38 , 3567 – 3576 .
- Kassaee , M.Z. ; Rostamizadeh , S. ; Shadjou , N. ; Motamedi , E. ; Esmaeelzadeh , M. J. Heterocyclic Chem . 2010 , 47 , 1421 – 1424 .
- Shaterian , H.R. ; Oveisi , A.R. ; Honarmand , M. Synth. Commun . 2010 , 40 , 1231 – 1242 .
- Shaabani , A. ; Rahmati , A. ; Moghimirad , J. C. R. Chimie 2008 , 11 , 759 – 764 Shaabani, A.; Rahmati, A.; Moghimirad, J. J. Heterocyclic Chem. 2008, 45, 1629–1632
- Li , C.J. Chem. Rev . 2005 , 105 , 3095 – 3165 .
- Gajewski , J.J. Acc. Chem. Res . 1997 , 30 , 219 – 225 .
- Pratt , L.R. ; Pohrille , A. Chem. Rev . 2002 , 102 , 2671 – 2692 .
- Ludwig , L. Angew Chem Int. Ed . 2001 , 40 , 1808 – 1827 .
- Khurana , J.M. ; Kumar , S. Tetrahedron Lett . 2009 , 50 , 4125 – 4127 .