Abstract
Heterogeneous Tin (IV) hydrogen phosphate nanodisks [Sn(HPO4)2.H2O] efficiently catalyzed the one-pot three component condensation of aromatic aldehydes, β-ketoesters and urea to produce 3,4–dihydropyrimidin-2-ones under solvent-free conditions at room temperature. Also, the catalyst is equally applicable for the preparation of 1,5–benzodiazepines under the same reaction conditions. The optimum load of the catalyst required is 10 mole% and reusable. Hence, the process is green.
Introduction
The development of green technology has received tremendous attention from chemists due to conservation of global eco-system Citation1. In this context, the development of new catalysts that are environmentally benign and effective are of great interests. Nanomaterials Citation2–4 with larger surface area and high density active sites to those of bulk materials have gained much interest in the field of chemistry for their catalytic properties in many important organic reactions. And, the reactions conducted under solvent-free conditions using reusable nano-catalysts Citation5–7 is now become a trust area of research in developing environmentally benign technology. In this endeavor, organic group transformation reactions using nanoparticles in organic solvents, for example, Ni Citation8, Cu Citation9, Ag Citation10, polysilane-supported Pd and Pt nanoparticles Citation11, RuO2-FAU nanoclusters Citation12, optically active bisphosphine (BINAP) stabilized Pd Citation13 are reported.
On the other hand, green synthesis of chiral Citation14 or achiral compounds through multicomponent reactions (MCRs) Citation15–17 reactions covers a major area of research in recent years. Multicomponent Biginelli reaction Citation18 offers an efficient route to access 3,4-dihydropyrimidin-2-ones (DHPMs) that show a wide range of important pharmacological properties like calcium channel blockers, antihypertensive agents, α-1a antagonist, neuropeptide Y (NPY) antagonists, anticancer drugs, and so on and alkaloids containing dihydropyrimidinone moiety particularly the batzelladine alkaloids are potent HIVgp-120-CD4 inhibitors Citation19–22.
Dihydropyrimidinones (DHPMs) scaffolds are synthesized by several improved protocols that involve bulk catalysts viz. Lewis and Brønsted acid catalysts Citation23–28, polymer supported reagents Citation29 Citation30, microwave-assisted methodologies Citation31, grinding techniques Citation32, and ultrasonic methods Citation33 in organic solvents. Recently, solvent-free conditions using Yb(OTf)3 Citation34, montmorillonite Citation35, and ionic liquid Citation36 as catalysts are reported where bulk amount of catalysts or rare earth metals are involved.
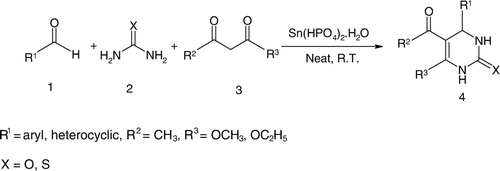
In continuation of our efforts toward the development of green synthetic methodologies Citation37–41 and nano materials Citation42, we now wish to report herein a highly efficient, green, and recyclable Tin (IV) hydrogen phosphate [Sn(HPO4)2.H2O] nanodisks Citation43 as heterogeneous catalyst for the synthesis of DHPMs under solvent-free condition at room temperature ().
Results and discussion
Initially, the one pot experiment of the Biginelli condensation was carried out with benzaldehyde, ethylacetoacetate, and urea catalyzed by Sn(HPO4)2.H2O nanodisks under solvent-free condition at room temperature. The optimum reaction condition was screened by using different catalyst loading under solvent-free conditions at room temperature and it was found that 10 mol% of the catalyst was the optimum load for the highest formation of DHPMs in the reaction mixture (, entry 3). An increase in the amount of the catalyst loading from 10 to 20 mol% gave almost the same yield of the product (). Having optimized the reaction conditions, a wide range of structurally varied aldehydes, β-ketoesters, and urea were coupled together by this protocol to produce the corresponding dihydropyrimidinones. The electronic variation (electron withdrawing and donating groups) on aryl aldehydes caused no appreciable change in the efficiency of the condensation. Thiourea was used with similar success to produce the corresponding dihydropyrimidin-2-thiones (, entries 13–15) that are also of much important with regard to biological activity Citation44. The catalyst was recovered quantitatively from the reaction mixture by simple filtration. After washed with ethanol (2 × 5 ml), it was dried in an oven at 60°C for 1 hour and reused for five consecutive times without loss of catalytic activity ().
Table 1. Synthesis of dihydropyrimidin-2(1H)-ones catalyzed by Sn(HPO4)2.H2O nanodisks with different catalyst loadings under solvent-free condition.
Table 2. Synthesis of dihydropyrimidin-2(1H)-ones and thiones catalyzed by Sn(HPO4)2.H2O nanodisks under solvent-free condition at room temperature.
Table 3. Recycling of Sn(HPO4)2.H2O nanodisks catalyst.
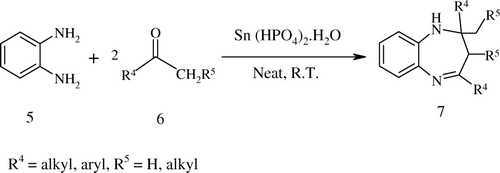
Having successfully established the methodology for the DHPMs scaffold, we were keen to explore the efficiency of the catalyst for the condensation of o-phenylenediamine with a ketone having an α-hydrogen under solvent-free conditions at room temperature () and gratifyingly, the respective 1,5-benzodiazepines Citation45–49 were produced with excellent yields (). The scope of the protocol was examined with o-phenylenediamine and different aliphatic (acyclic and cyclic) and aromatic ketones and it formed 1,5-benzodiazepines in excellent yields within 1.2–2 hours using 10 mol% of the catalyst under the same reaction conditions.
Table 4. Synthesis of 1,5-benzodiazepines catalyzed by Sn(HPO4)2.H2O nanodisks under solvent-free condition at room temperature.
Experimental
All reagents were obtained from commercial sources and used without further purification. The solvents for chromatography were distilled before use. Melting points were measured using Buchi B-540 apparatus and are uncorrected. 1H NMR spectra were recorded on Avance DPX 300 MHz FT-NMR spectrometer. Chemical shifts are expressed in Δ units relative to tetramethylsilane (TMS) signal as internal reference in DMSO-d6 or CDCl3. The Fourier transform-infra red (FT-IR) spectra were recorded on FT-IR-system-2000 Perkin Elmer spectrometer in CHCl3 or on KBr pellets. The SEM was recorded by Scanning Electron Microscope Model: JEOL JSM-6390LV. The X-ray diffraction (XRD) patterns were recorded on a Rigaku Miniflex diffractometer with monochromatized Cu Kα radiation (λ = 1.5418 Å). Gas chromatography (GC) analysis was performed on the Chemito GC 1000 spectrometer.
General procedure for the synthesis of 3, 4-dihydropyrimidin-2-ones
In a 50 ml round-bottom flask, aldehyde (2 mmol), ethyl aetoaetate (2 mmol), and urea (3 mmol) were stirred in presence of Sn(HPO4)2.H2O nanodisks Citation43 (10 mol%) under solvent-free condition at room temperature for the stipulated time. The progress of the reaction was monitored by TLC. After completion of the reaction, methanol was added to dissolve the solid formed and the catalyst was filtered off, the filtrate was concentrated in vacuum and recrystallized from ethanol to afford pure product.
General procedure for the synthesis of 1, 5-benzodiazepines
In a 50 ml round-bottom flask, o-phenylenediamines (1mmol) and ketones (2.2 mmol) were stirred in presence of Sn(HPO4)2.H2O nanodisks (10 mol%) under solvent-free condition at the room temperature. The reaction was monitored by TLC. After completion of the reaction, ethyl acetate was added, catalyst was filtered off, and the products were purified by column chromatography on silica gel (60–120 mesh) using ethyl acetate/hexane mixture as eluent to afford pure 1, 5-benzodiazepines.
5-(Ethoxycarbonyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (, entry 1)
M.P. 202–203°C; 1H NMR (DMSO, 300 MHz): Δ 1.08 (t, 3H), 2.23 (s, 3H), 3.98 (q, 2H), 5.13 (d, 1H), 7.10–7.29 (m, 5H), 7.81 (s, 1H), 9.15 (s, 1H); FT-IR (KBr, cm–1): 1635.2, 1725.1, 3240.5; MS (m/z): 260.26 (M + ); Anal. Calcd. for C14H16O3N2: C, 64.60; H, 6.20; N, 10.76. Found: C, 64.56; H, 6.14; N, 10.74.
5-(Ethoxycarbonyl)-6-methyl-4-(4-methoxyphenyl)-3,4-dihydropyrimidin-2(1H)-thione (, entry 14)
M.P. 150–151°C; 1H NMR (DMSO, 300 MHz): Δ 1.09 (t, 3H), 2.26 (s, 3H), 3.72 (s, 3H), 3.99 (q, 2H), 5.11 (s, 1H), 6.88–7.16 (m, 4H), 9.49 (s, 1H), 10.21 (s, 1H); FT-IR (KBr, cm–1): 1561.4, 1599.0, 1651.2, 3250.8; MS (m/z): 306.32 (M + ); Anal. Calcd. For C15H18O3N2S: C, 58.80; H, 5.92; N, 9.14. Found: C, 58.74; H, 5.97; N, 9.19.
2-Methyl-2,4-diisobutyl-2,3-dihydro-1H-1,5-benzodiazepine (, entry 5)
M.P. 118–120°C; 1H NMR (CDCl3, 300 MHz): Δ 0.95–1.05 (m, 12H), 1.33 (s, 3H), 1.49–1.52 (m, 2H), 1.65–1.75 (m, 1H), 2.05–2.25 (m, 3H), 2.24 (d, 2H), 6.60–6.65 (m, 1H), 6.85–6.95 (m, 2H), 7.05–7.15 (m, 1H); FT-IR (KBr, cm–1): 3330.7, 1637.5; MS (m/z): 272.32 (M + ); Anal. Calcd. For C18H28N2: C, 79.36; H, 10.36; N, 10.28. Found: C, 79.27; H, 10.23; N, 10.19.
Conclusion
The catalytic activity of Sn(HPO4)2.H2O nanodisks as a heterogeneous catalyst for the synthesis of 3,4-dihydropyrimidin-2-ones under solvent-free condition at room temperature was investigated. Also, the catalyst was found to be equally effective for the synthesis of 1,5-benzodiazepines. The nanodisks behave as a Brønsted acid under the reaction conditions. Also, the catalyst is reusable, the reaction is multicomponent one pot synthesis under solvent-free conditions and, hence, it is green.
Supporting information
All the products were fully characterized by 1H and 13C NMR and MS analyses. The experimental procedures and spectral data of the compounds are available in the Supporting Information (Appendix 1).
Acknowledgements
The authors thank the Director, Dr. P.G. Rao, HOD, Dr. J.C.S. Kataky, Synthetic Organic Chemistry Division and the Analytical Division of NEIST, Jorhat, Assam, India, for their help. P.H. thanks CSIR, New Delhi, for the research fellowship.
References
- Anastas , P.T. ; Heine , L.G. ; Williamson , T.C. Introduction . In Green Chemical Syntheses and Processes ; Anastas , P.T. , Heine , L.G. , Williamson , T.C. , Washington, DC : American Chemical Society , 2000 ; Chapter 1-6.
- Goldstein , A.N. 1997 . Handbook of Nanophase Materials , Marcel Dekker : New York .
- Lu , L. , Sui , M.L. and Lu , K. 2000 . Science 1463
- Schmid , G. 2004 . Nanoprticles , Weinheim, , Germany : Wiley-VCH .
- Bruss , A.J. , Gelesky , M.A. , Machadoa , G.P. and Dupont , J. J. 2006 . Mol. Catal 212
- Adams , R.D. , Boswell , E.M. , Captain , B.A. , Hungria , B. , Midgley , P.A. , Raja , R. and Thomas , J.M. 2007 . Angew. Chem Int , Ed 8182
- Hu , J. , Chen , L. , Zhu , K. , Suchopar , A. and Richards , R. 2007 . Catalysis Today 277
- Saxena , A. , Kumar , A. and Majumdar , S. 2007 . Appl. Catal. A. General 210
- Kidwai , M. , Bansal , V. , Saxena , A. , Aerry , S. and Mazumdar , S. 2006 . Tetrahedron Lett 8049
- Yan , W. , Wang , R. , Xu , Z. , Xu , J. , Lin , L. , Shen , Z. and Zhou , Y. J. 2006 . Mol. Catal. A 81
- Oyamada , H. , Akiyama , R. , Hagio , H. , Naito , T. and Kobayashi , S. 2006 . Chem. Commun 4297
- Zhan , B.-Z. , White , M.A. , Sham , T.-K. , Pincock , J.A. , Doucet , R.J. , Ramana Rao , K.V. , Robertson , K.N. and Cameron , T.S. 2003 . J. Am. Chem. Soc 2195
- Tatumia , R. ; Akitab , T. ; Fujihara , H. Chem. Commun . 2006 , 31 , 3349 .
- Huang , Y. ; Yangm F. ; Zhu , C. Am. Chem. Soc ., 2005 , 47 , 16386 .
- Zhu , J. ; Bienayme , H. , Eds . Multicomponent Reactions ; Wiley-VCH : Weinheim, Germany 2005 .
- Ramon , D.J. ; Yus , M. Angew. Chem., Int . Ed . 2005 , 44 , 1602 .
- Burke , M.D. ; Schreiber, S.L. Angew. Chem., Int . Ed . 2004 , 43 , 46 .
- Biginelli , P. Gazz. Chim. Ital . 1893 , 23 , 360 .
- Atwal , K.S. ; Rovnyak , G.C. ; Kimball , S.D. ; Floyd , D.M. ; Moreland , S. ; Swanson , B.N. ; Gougoutas , J.Z. ; Schwartz , J. ; Smillie , K.M. ; Malley , M.F. J. Med. Chem . 1990 , , 2629 33
- Atwal , K.S. ; Swanson , B.N. ; Unger , S.E. ; Floyd , D.M. ; Moreland , S. ; Hedberg , A. ; O'Reilly , B.C. J. Med. Chem . 1991 , 34 , 806 and references cited therein.
- Heys , L. ; Moore , C.G. ; Murphy , P.J. Chem. Soc. Rev . 2000 , 29 , 57 .
- Aron , Z.D. ; Overman , L.E. Chem. Commun . 2004 , 253 .
- Hu , E.H. ; Sidler , D.R. ; Dolling , U.-H. J. Org. Chem . 1998 , 10 , 3454 .
- Ranu , B.C. ; Hajra , A. ; Jana , U. J. Org. Chem . 2000 , 65 , 6270 .
- Bose , D.S. ; Fatima , L. ; Mereyala , H.B. J. Org. Chem . 2003 , 68 , 587 .
- Wang , Z.-T. ; Xu , L.-W. ; Xia , C.-G. ; Wang , H.-Q. Tetrahedron Lett . 2004 , 45 , 7951 .
- Heravi , M.M. ; Bakhtiari , K. ; Bamoharram , F.F. Catal. Commun . 2006 , 7 , 373 .
- Debache , A. ; Boumoud , B. ; Amimour , M. ; Belfaitah , A. ; Rhouati , S. ; Carboni , B. Tetrahedron Lett . 2006 , 47 , 5697 .
- Dondoni , A. ; Massi , A. Tetrahedron Lett . 2004 , 42 , 7975 .
- Palaniappan , S. ; John , A. J. Mol. Catal. A: Chem . 2005 , 233 , 9 .
- Gohain , M. ; Prajapati , D. ; Sandhu , J.S. Synlett . 2004 , 235 .
- Ranu , B.C. ; Hajra , A. ; Dey , S.S. Org. Process Res. Develop . 2002 , 6, 817 .
- Zhang , X. ; Li , Y. ; Liu , C. ; Wang , J. J. Mol. Catal. A: Chem . 2006 , 253, 207 .
- Ma , Y. ; Qian , C. ; Wang , L. ; Yang , M. J. Org. Chem . 2000 , 65, 3864 .
- Bigi , F. ; Carloni , S. ; Frullanti , B. ; Maggi , R. ; Sartori , G. Tetrahedron Lett . 1999 , 40, 3465 .
- Peng , J. ; Deng , Y. Tetrahedron Lett . 2001 , 42, 5917 .
- Gogoi , P. ; Sarmah , G.K. ; Konwar , D. J. Org. Chem . 2004 , 69, 5153 .
- Gogoi , P. Hazarika , P. ; Konwar , D. J. Org. Chem . 2005 , 70, 1934 .
- Das Sharma , S. ; Gogoi , P. ; Konwar , D. Green Chem . 2007 , 9, 153 .
- Gogoi , P. ; Konwar , D. Tetrahedron Lett . 2006 , 47, 79 .
- Gogoi , P. ; Konwar , D. Org. Biomol. Chem . 2005 , 3, 3473 .
- Hazarika , P. ; Das Sharma , S. ; Konwar , D. Catal. Commun . 2008 , 9, 2398.
- Qiao , H. ; Jia , F. ; Ai , Z. ; Li , Z. ; Zhang , L. Chem. Commun . 2006 , 2033 .
- Kappe , C.O. Tetrahedron , 1993 , 49, 6937 .
- Schutz , H. Benzodiazepines ; Weinheim, , Germany : Springer , 1982 ; Vol. 2, p 240 .
- Sternbach , L.H. J. Med. Chem . 1979 , 22, 1 .
- Römer , D. ; Büscher , H.H. ; Hill , R.C. ; Maurer , R. ; Petcher , T.J. ; Zeugner , R.H. ; Benson , W. ; Finner , E. ; Milkowski Thies , W.P.W. Nature 1982 , 298, 759 .
- Merluzzi , V.J. ; Hargrave , K.D. ; Labadia , M. ; Grozinger , K. ; Skoog , M. ; Wu , J.C. ; Shih , C.K. ; Eckner , K. ; Hattox , S. ; Adams , J. , Science 1990 , 250, 411
- Hazarika , P. ; Gogoi , P. ; Konwar , D. Syn. Commun . 2007 , 37, 3447 .
Appendix 1: Supporting information
Experimental part
General information
All reagents were obtained from commercial sources and used without further purification. The solvents for chromatography were distilled before use. Melting points were measured using Buchi B-540 apparatus and are uncorrected. 1H NMR spectra were recorded on Avance DPX 300 MHz FT-NMR spectrometer. Chemical shifts are expressed in Δ units relative to tetramethylsilane (TMS) signal as internal reference in DMSO-d6 or CDCl3. FT-IR spectra were recorded on FT-IR-system-2000 Perkin Elmer spectrometer in CHCl3 or on KBr pellets. SEM was recorded by Scanning Electron Microscope model JEOL JSM-6390LV. X-ray diffraction (XRD) patterns were recorded on a Rigaku Miniflex diffractometer with monochromatized Cu Kα radiation (λ = 1.5418 Å). Gas chromatography (GC) analysis was performed on Chemito GC 1000 Spectrometer.
Preparation of Sn(HPO4)2.H2O nanodisks
Sn(HPO4)2.H2O nanodisks were prepared by following the published procedure Citation1. H3PO4 (85%) (0.3 ml) was added to a solution of 0.35g of SnCl4.5H2O (AR) in anhydrous ethanol (12 ml). The resulting reaction mixture was transferred to a 22 ml teflon-sealed autoclave and stored at 180°C for 24 hours, then air-cooled to room temperature. The product was washed several times with anhydrous ethanol and finally dried at 60°C in a vacuum oven. The catalyst was characterized by SEM, XRD, and FT-IR analyses.
Reference
1. Qiao, H.; Jia, F.; Ai, Z.; Li, Z.; Zhang, L. Chem. Commun. 2006, 2033.
General procedure for the formation of 3,4-dihydropyrimidin-2-ones:
In a 50 ml round-bottom flask, aldehyde (2 mmol), ethyl aetoacetate (2 mmol) and urea (3 mmol) were stirred in presence of Sn(HPO4)2.H2O nanodisks (10 mol%) under solvent-free condition at room temperature for the stipulated time. The progress of the reaction was monitored by TLC. After completion of the reaction, methanol was added to dissolve the solid formed and the catalyst was filtered off, the filtrate was concentrated in vacuum and recrystallized from ethanol to afford pure product.
Spectral data of the 3,4-dihydropyrimidin-2-ones and thiones obtained by the condensation of aldehydes,1,3-dicarbonyls and urea/thiourea
5-(Ethoxycarbonyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (
, entry 1)
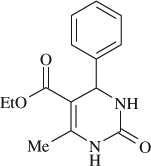
M.P. 202–203°C; 1H NMR (DMSO, 300 MHz): Δ1.08 (t, 3H), 2.23 (s, 3H), 3.98 (q, 2H), 5.13 (d, 1H), 7.10–7.29 (m, 5H), 7.81 (s, 1H), 9.15 (s, 1H); FT-IR (KBr, cm–1): 1635.2, 1725.1, 3240.5; MS (m/z): 260.26 (M + ); Anal. Calcd. for C14H16O3N2:C, 64.60; H, 6.20; N, 10.76. Found: C, 64.56; H, 6.14; N, 10.74.
5-(ethoxycarbonyl)-4-(4-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (
, entry 2)
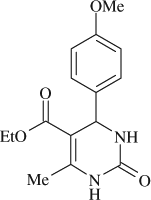
M.P. 200–201°C; 1H NMR (DMSO, 300 MHz): Δ1.10 (t, 3H), 2.25 (s, 3H), 3.73 (s, 3H), 3.98 (q, 2H), 5.16 (s, 1H), 7.01–7.21 (m, 4H), 7.73 (s, 1H), 9.19 (s, 1H); FT-IR (KBr, cm–1): 1635.7, 1717.5, 3242.1; MS (m/z): 290.39 (M + ); Anal. Calcd. for C15H18O4N2:C, 62.06; H, 6.25; N, 9.65. Found: C, 62.11; H, 6.16; N, 9.72.
5-(ethoxycarbonyl)-6-methyl-4-(4-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one (
, entry 3)
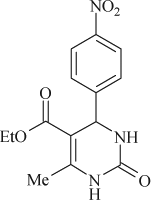
M.P. 208–209°C; 1H NMR (DMSO, 300 MHz): Δ1.09 (t, 3H), 2.24 (s, 3H), 3.76 (q, 2H), 5.19 (d, 1H), 7.62–8.10 (m, 4H), 7.92 (s, 1H), 9.28 (s, 1H); FT-IR (KBr, cm–1): 1644.2, 1723.6, 3240.0; MS(m/z): 305.20 (M + ); Anal. Calcd. for C14H15O5N3:C, 55.08; H, 4.95; N, 13.76. Found: C, 55.17; H, 4.88; N, 13.62.
5-(ethoxycarbonyl)-6-methyl-4-(4-chlorophenyl)-3,4-dihydropyrimidin-2(1H)-one (
, entry 4)
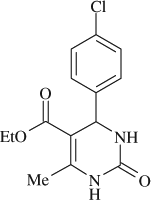
M.P. 210–211°C; 1H NMR (DMSO, 300 MHz): Δ1.10 (t, 3H), 2.25 (s, 3H), 3.89 (q, 2H), 5.16 (s, 1H), 7.31–7.50 (m, 4H), 7.80 (s, 1H), 9.25 (s, 1H); FT-IR (KBr, cm–1): 1644.0, 1723.1, 3239.7; MS(m/z): 294.79 (M + ); Anal. Calcd. for C14H15O3N2Cl:C, 57.05; H, 5.13; N, 9.50. Found: C, 57.12; H, 5.04; N, 9.45.
5-(ethoxycarbonyl)-4-(2,4-dichlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (
, entry 5)
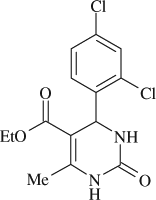
M.P. 238–240°C; 1H NMR (DMSO, 300 MHz): Δ1.00 (t, 3H), 2.29 (s, 3H,), 3.90 (q, 2H), 5.60 (s, 1H), 7.32 (d, 1H), 7.42 (d, 1H), 7.57 ((s, 1H), 7.77 (s, 1H), 9.33 (s, 1H); FT-IR (KBr, cm–1): 1635, 1697, 3357; MS (m/z): 328.80 (M + ); Anal. Calcd. For C14H14O3N2Cl2:C, 51.09; H, 4.25; N, 8.51; Found: C, 51.12; H, 4.21; N, 8.52.
5-(ethoxycarbonyl)-4-(3-hydroxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (
, entry 6)
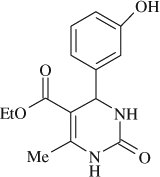
M.P. 163–164°C; 1H NMR (DMSO, 300 MHz): Δ1.09 (t, 3H), 2.22 (s, 3H), 4.01 (q, 2H), 5.05 (d, 1H), 6.6 (s, 1H), 6.51–7.06 (m, 4H), 7.71 (s, 1H), 9.17 (s, 1H), 9.36 (s, 1H); FT-IR (KBr, cm–1): 1644.2, 1723.6, 3240.0; MS (m/z): 276.23 (M + ); Anal. Calcd. For C14H16O4N2:C, 60.86; H, 5.84; N, 10.14. Found: C, 60.79; H, 5.88; N, 10.10.
5-(ethoxycarbonyl)-6-methyl-4-styryl-3,4-dihydropyrimidin-2(1H)-one (
, entry 7)
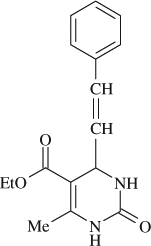
M.P. 232–235°C; 1H NMR (DMSO, 300 MHz): Δ1.20 (t, 3H), 2.21 (s, 3H), 4.09 (m, 2H), 4.74 (d, 1H), 6.20 (dd, 1H), 6.37 (d, 1H), 7.21–7.46 ( m, 5H), 7.53 (d, 1H), 9.13(s, 1H); MS (m/z): 286.27 (M + ); Anal. Calcd. for C16H18O3N2:C, 67.12; H, 6.33; N, 9.78; Found: C, 67.09; H, 6.35; N, 9.75.
5-(ethoxycarbonyl)-4-(iso-propyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (
, entry 8)
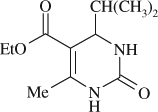
M.P. 170–171°C; 1H NMR (DMSO, 300 MHz): Δ0.76–0.81 (m, 6H), 1.07 (t, 3H), 1.69 (m, 1H), 2.19 (s, 3H), 4.02 (q, 2H), 4.89 (s, 1H), 7.54 (s, 1H), 9.00 (s, 1H); FT-IR (KBr, cm–1): 1642.0, 1709.5, 3235.0; MS (m/z): 226.21 (M + ); Anal. Calcd. for C11H18O3N2:C, 58.39; H, 8.02; N, 12.38. Found: C, 58.33; H, 8.08; N, 12.30.
5-(ethoxycarbonyl)-4-(2-furyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (
, entry 9)
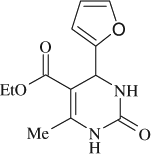
M.P. 203–204°C; 1H NMR (DMSO, 300 MHz): Δ1.12 (t, 3H), 2.21 (s, 3H), 4.02 (q, 2H), 5.18 (d, 1H), 6.4–7.5 (m, 3H), 7.76 (s, 1H), 9.20 (s, 1H); FT-IR (KBr, cm–1): 1644.0, 1706.1, 3240.2; MS (m/z): 250.29 (M + ); Anal. Calcd. for C12H14O4N2:C, 57.59; H, 5.64; N, 11.19. Found: C, 57.65; H, 5.70; N, 11.27.
5-(methoxycarbonyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (
, entry 10)
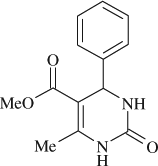
M.P. 207–208°C; 1H NMR (DMSO, 300 MHz): Δ2.21 (s, 3H), 3.56 (s, 3H), 5.18 (d, 1H), 7.26–7.33 (m, 5H), 7.78 (s, 1H), 9.23 (s, 1H); FT-IR (KBr, cm–1): 1642.0, 1701.1, 3231.8; MS (m/z): 246.21 (M + ); Anal. Calcd. for C13H14O3N2:C, 63.40; H, 5.73; N, 11.38. Found: C, 63.33; H, 5.79; N, 11.32.
5-(mehoxycarbonyl)-4-(4-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (
, entry 11)
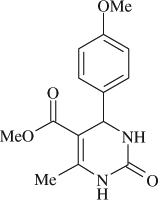
M.P. 207–208°C; 1H NMR (DMSO, 300 MHz): Δ2.24 (s, 3H), 3.53 (s, 3H), 3.72 (s, 3H), 5.09 (d, 1H), 6.88 (d, 2H), 7.14 (d, 2H), 7.69 (s, 1H), 9.19 (s, 1H); FT-IR (KBr, cm–1): 1645, 1697, 3360; MS (m/z): 276.22 (M + ); Anal. Calcd. for C14H16O4N2:C, 60.87; H, 5.83; N, 10.14; Found: C, 60.85; H, 5.86; N, 10.16.
5-(methoxycarbonyl)-6-methyl-4-(4-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one (
, entry 12)
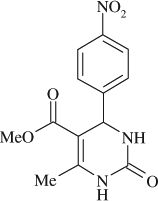
M.P. 235–236°C; 1H NMR (DMSO, 300 MHz): Δ2.26 (s, 3H), 3.52 (s, 3H), 5.31 (d, 1H), 7.53–8.19 (m, 4H), 7.89 (s, 1H), 9.31 (s, 1H); FT-IR (KBr, cm–1): 1692.8, 1712.6, 3232.4; MS (m/z): 291.21 (M + ); Anal. Calcd. for C13H13O5N3:C, 53.61; H, 4.50; N, 14.43. Found: C, 53.56; H, 4.53; N, 14.37.
5-(ethoxycarbonyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione (
, entry 13)
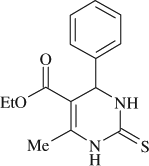
M.P. 207–209°C; 1H NMR (DMSO, 300 MHz): Δ1.09 (t, 3H), 2.27 (s, 3H), 3.99 (q, 2H), 5.15 (d, 1H), 7.20–7.35 (m, 5H), 9.63 (s, 1H), 10.29 (s, 1H); FT-IR (KBr, cm–1): 1578.3, 1672.7, 3181.2, 3339.8; MS (m/z): 276.31 (M + ); Anal. Calcd. For C14H16O2N2S:C, 60.85; H, 5.84; N, 10.14. Found: C, 60.78; H, 5.90; N, 10.08.
5-(ethoxycarbonyl)-6-methyl-4-(4-methoxyphenyl)-3,4-dihydropyrimidin-2(1H)-thione (
, entry 14)
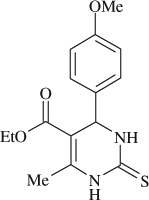
M.P. 150–151°C; 1H NMR (DMSO, 300 MHz): Δ1.09 (t, 3H), 2.26 (s, 3H), 3.72 (s, 3H), 3.99 (q, 2H), 5.11 (s, 1H), 6.88–7.16 (m, 4H), 9.49 (s, 1H), 10.21 (s, 1H); FT-IR (KBr, cm–1): 1561.4, 1599.0, 1651.2, 3250.8; MS (m/z): 306.32 (M + ); Anal. Calcd. For C15H18O3N2S:C, 58.80; H, 5.92; N, 9.14.Found: C, 58.74; H, 5.97; N, 9.19.
5-(ethoxycarbonyl)-6-methyl-4-(4-Chlorophenyl)-3,4-dihydropyrimidin-2(1H)-Thione (
, entry 15)
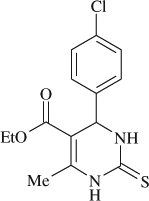
M.P. 183–185°C; 1H NMR (CDCl3, 300 MHz): Δ1.21 (t, 3H), 2.38 (s, 3H), 4.12 ( q, 2H), 5.40 (s, 1H), 7.15 (s, 1H), 7.27 (d, 2H), 7.33 (d, 2H), 7.68(s, 1H); FT-IR (KBr, cm–1): 3329, 3174, 3103, 2983, 2935, 2360, 1722, 1573, 1465, 1371, 1282, 1203, 1093, 806, 746; MS (m/z): 310.66 (M + ); Anal. Calcd. For C14H15O2N2SCl: C, 54.10, H, 4.86; N, 9.01; Found: C, 54.07, H, 4.88, N, 9.03.
General Procedure for the formation of 1,5-benzodiazepines
In a 50 ml round-bottom flask, o-phenylenediamines (1 mmol) and ketones (2.2 mmol) were stirred in presence of Sn(HPO4)2.H2O nanodisks (10 mol%) under solvent-free condition at the room temperature. The reaction was monitored by TLC. After completion of the reaction, ethyl acetate was added, catalyst was filtered off, and the products were purified by column chromatography on silica gel (60–120 mesh) using ethyl acetate/hexane mixture as eluent to afford pure 1,5-benzodiazepines.
Spectral data of the 1,5-benzodiazepines obtained by the condensation of ketone and 1,2-diamines
2,2,4-trimethyl-2,3-dihydro-1H-1,5-benzodiazepine (
, entry 1)
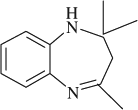
M.P. 136–137°C; 1H NMR (CDCl3, 300 MHz): Δ1.35 (s, 6H), 2.21 (s, 2H), 2.35 (s, 3H), 3.45 (s, 1H), 6.62–7.31 (m, 4H); FT-IR (KBr, cm–1): 3340.4, 1650.8; MS (m/z): 188.21 (M + ); Anal. Calcd. For C12H16N2:C, 76.55; H, 8.57; N, 14.88. Found: C, 76.49; H, 8.51; N, 14.80.
2,2,4-triethyl-3-methyl-2,3-dihydro-1H-1,5-benzodiazepine (
, entry 2)
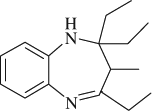
M.P. 143–144°C; 1H NMR (CDCl3, 300 MHz): Δ0.75–1.05 (m, 10H), 1.20–1.40 (m, 4H), 1.48–1.64 (m, 2H), 2.40–2.59 (m, 2H), 2.87 (q, 1H), 3.73 (s, 1H), 6.55 (d, 1H), 6.64 (t, 1H), 6.90 (t, 1H), 7.36 (d, 1H); FT-IR (KBr, cm–1): 3321.0, 1638.2; MS (m/z): 244.29 (M + ); Anal. Calcd. For C16H24N2:C, 78.64; H, 9.90; N, 11.46. Found: C, 78.54; H, 9.80; N, 11.31.
2,4-diethyl-2-methyl-2,3-dihydro-1H-1,5-benzodiazepine (
, entry 3)
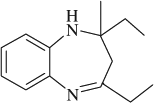
M.P. 137–138°C; 1H NMR (CDCl3, 300 MHz): Δ0.97 (t, 3H), 1.26 (t, 3H), 1.72 (q, 2H), 2.15 (m, 2H), 2.34 (s, 3H), 2.69 (q, 2H), 3.45 (s, 1H), 6.65–7.33 (m, 4H); FT-IR (KBr, cm–1): 3332.0, 1638.5; MS (m/z): 216.27 (M + ); Anal. Calcd. For C14H20N2: C, 77.73; H, 9.32; N, 12.95. Found: C, 77.65; H, 9.24; N, 12.83.
2-methyl-2,4-diphenyl-2,3-dihydro-1H-1,5-benzodiazepine (
, entry 4)
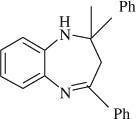
M.P. 150–151°C; 1H NMR (CDCl3, 300 MHz): Δ1.80 (s, 3H), 2.95 (d, 1H), 3.16 (d, 1H), 3.44 (s, 1H), 6.53–7.02 (m, 3H), 7.16–7.34 (m, 7H), 7.55–7.65 (m, 4H); FT-IR (KBr, cm–1): 3330.9, 1635.2; MS (m/z): 312.48 (M + ); Anal. Calcd. For C22H20N2:C, 84.58; H, 6.45; N, 8.97. Found: C, 84.51; H, 6.38; N, 8.91.
2-methyl-2,4-diisobutyl-2,3-dihydro-1H-1,5-benzodiazepine (
, entry 5)
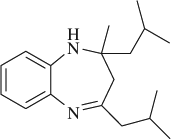
M.P. 118–120°C; 1H NMR (CDCl3, 300 MHz): Δ0.95–1.05 (m, 12H), 1.33 (s, 3H), 1.49–1.52 (m, 2H), 1.65–1.75 (m, 1H), 2.05–2.25 (m, 3H), 2.24 (d, 2H), 6.60–6.65 (m, 1H), 6.85–6.95 (m, 2H), 7.05–7.15 (m, 1H); FT-IR (KBr, cm–1): 3330.7, 1637.5; MS (m/z): 272.32 (M + ); Anal. Calcd. For C18H28N2:C, 79.36; H, 10.36; N, 10.28. Found: C, 79.27; H, 10.23; N, 10.19.
10-spirocyclohexane-2,3,4,10,11,11a-hexahydro-1H-dibenzo[b,e][1,4]diazepine (
, entry 6)
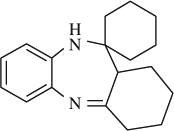
M.P. 136–137°C; 1H NMR (CDCl3, 300 MHz): Δ1.23–1.86 (m, 16H), 2.30–2.70 (m, 3H), 4.47 (s, 1H), 6.69–7.70 (m, 4H); FT-IR (KBr, cm–1): 3290.9, 1640.2; MS (m/z): 268.24 (M + ); Anal. Calcd. for C18H24N2: C, 80.55; H, 9.01; N, 10.44. Found: C, 80.46; H, 9.10; N, 10.35.