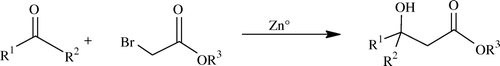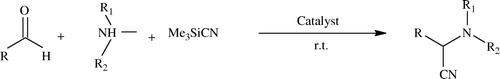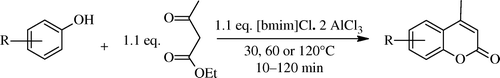Abstract
In the second part of our paper, further recent developments of ionic liquids in selected name reactions of carbonyl chemistry such as Mannich, Reformatsky, Cannizaro, Streacker, Barbier, Pechmann, etc. are described.
Introduction
Ionic liquids (ILs) are implicated in both experimental scientific studies and commercial production. This is necessitated because of environmental concerns specific to the present century; imposition of these concerns is manifested through enactment of various regulations/laws by various countries Citation1. Not only are these prohibited through legal actions but also some incentives are given in the form of awards and prizes to researchers Citation2–6. Organic chemists were naturally attracted to the synthesis as well as use of Ils, and significant efforts have been made in the study of major named reactions of organic chemistry; therefore, ILs have been tested for many named reactions of organic chemistry, due to their attractive properties such as: they are not volatile and therefore not harmful to environment; hence, green solvents are replacing volatile organic solvents and unsafe catalysts. They also obey the 12 principles of green chemistry such as atom economy, recyclability, safety etc., Citation7–21. Use of ILs at a mega/industrial scale is still a challenge, but if they are successfully produced at large scale the future of life on earth will be safe from air, water, and soil pollution. But in spite of these advantages, one question remains unanswered, namely whether the manufacture of ILs is green and whether it is in favor of ecosystems or not.
In the first part of this paper, the authors have reported developments in this direction using ILs as solvents in some selected named reactions of organic chemistry Citation22; some remaining ones are presented in this second part. The reactions selected here are given below, and in this paper the authors' own work is also incorporated:
| • | Mannich reaction | ||||
| • | Reformatsky reaction | ||||
| • | Streacker reaction | ||||
| • | Barbier reaction | ||||
| • | Pechmann reaction | ||||
| • | Henry reaction | ||||
| • | Cannizzaro reaction | ||||
Ionic liquids, considered as alternative solvents of the present century, are discussed in the following sections as well as their role/use in major reactions.
Mannich reaction
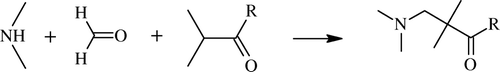
Mannich reaction is one of the most important carbon–carbon bond-forming reaction of organic chemistry for preparation of β-amino carbonyl compounds and 1,2-amino alcohol derivatives (). β-Amino-carbonyl compound formed from amino alkylation of α-carbon of carbonyl group with formaldehyde and ammonia or any primary or secondary amine is known as Mannich reaction, named after its discoverer, Carl Mannich Citation23. Reaction of aldimines and α-methylene carbonyls also give same products, which are valuable synthetic intermediates for the synthesis of drugs and biologically active compounds Citation24 Citation25. Some green production processes/procedures in this reaction, such as microwave Citation26 Citation27 or ultrasound irradiation Citation28 and the use of Lewis acids Citation29–31 have been reported. Also, Lewis bases Citation32, Brønsted acids Citation33–35, rare metal salts Citation36 Citation37, or organo catalysts Citation38–40 etc., do act as promoters to catalyze Mannich-type reactions. The present authors also did contributed to for a greener procedure development by using microwave irradiation Citation41.
Careful examination reveals that it is a simple nucleophilic addition of an amine to a carbonyl group followed by dehydration to the Schiff base, and this Schiff base acts an electrophile which reacts in the second step in a nucleophilic addition manner to carbonyl compound containing an acidic-proton.
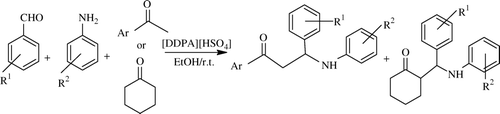
Use of IL greener solvents of present century was reported by Fang et al. in this reaction in a three-component way. So, here Mannich-type reactions of aromatic aldehydes, aromatic amines, and ketones which are catalyzed by a novel functionalized IL, 3-(N,N-dimethyldodecylammonium)propanesulfonic acid hydrogen sulfate ([DDPA][HSO4]) at room temperature in ethanol gave various b-amino carbonyl compounds in good yields; see Citation42. They recycled the catalyst at least six times without any catalyst activity reduction. Same groups used several ILs in this reaction to evaluate their efficacy, e.g. 3-(N,N-dimethyldodecyl ammonium) propanesulfonic acid hydrogen sulfate [DMDAPS][HSO4] Citation43, 3-(N,N-dimethyldodecylammonium)propanesulfonic acid hydrogen sulfate [DDPA][HSO4] Citation44, 1-butyl-3-methylimidazolium hydroxide ([bmim]OH) Citation45, 1-butyl-3-methylimidazolium hydroxide ([bmim][OH]) Citation46, 3-N,N,N-trimetylammoniumpropanesulfonic acid hydrogen sulfate Citation47, N,N,N-trimethyl-N-butanesulfonic acid ammonium hydrogen sulfate [TMBSA]HSO4 Citation48 . Invariably, these authors used these ILs in green solvents such as water and alcohol. Regarding scope reaction seems to be fairly general, as a large number of aldehydes have been used here.
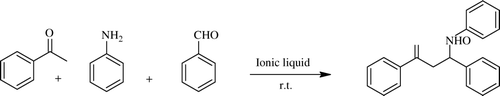
Juliana and co-workers reported application of several 1-butyl-3-methylimidazolium (BMIM) salt as ILs, i.e. [BMIM][NTf2] as solvent in the α-methylenation of carbonyl compounds at room temperature without any other catalyst (). This IL was reused without affecting the reaction rates or yields over seven runs Citation49. Rasalkar group describes Mannich reaction, catalyzed by phosphotungstic acid (H3PW12O40), a heteropoly acid in IL 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4) offered the best results in terms of yield of the products Citation50. Brønsted acidic IL containing nucleophile 1-methylimidazole and triphenylphosphine with 1,4-butane sultone and inorganic anions p-toluenesulfonic acid (PTSA) and trifluoroacetic acid (TFA) catalyzed Mannich reaction smoothly to afford β-amino carbonyl compounds in excellent yield and in shorter reaction time.
Here, IL is used as a catalyst as well as a solvent which was recycled seven times Citation51. Li et al. condensed aromatic aldehydes, anilines, and acetophenone and these were efficiently catalyzed by a recyclable carboxyl-functionalized IL [cmmim][BF4] and [bmim][BF4] in aqueous under mild conditions Citation52. The Mannich-type reaction of silyl enolates with aldimines proceeded smoothly with [emim]OTf as a solvent and without the addition of an activator to afford β-amino carbonyl compounds in excellent yields Citation53. IL media chosen by Liu and co-workers were l-butyl-3-methylimidazolium tetrafluoroborate (BMImBF4)/1-butyl-3-methylimidazolium dihydrogen phosphate (BMImH2PO4) and 1-ethylimidazolium trifluoroacetic acid (HEImTA) Citation54. 1,1,3,3-tetramethylguanidinium trifluoroacetate IL prepared by neutralization of 1,1,3,3-tetramethylguanidine with different acids under ambient condition and used by Gao co-workers in the synthesis of this reaction Citation55. In a similar way, acidic ILs as catalysts and solvents [Hmim]Tfa2 were used by the Zhao group and they obtained Mannich adducts in excellent yields Citation56. A mixture of aldehyde/imine, 3-buten-2-ol and RuCl2(PPh3)3 (0.005 mmol) in [bmim]PF6 (0.3 mL) was stirred at 90 °C Citation57 affords the desired products in very high yields. Another group reported use of ILs in benzene employing aniline, benzaldehyde, and acetophenone trimethylsilylenolate in solution of Yb(OTf)3 in [bmim][PF6]/benzne at 20 °C and stirred for 15 minutes to obtain products in excellent yields Citation58.
Asymmetric-Mannich reaction
Asymmetric version of this reaction is reported by Liu and co-workers who used amide ILs (AILs)/L-proline in synergy catalyzed Mannich reaction and reactants isovaleraldehyde, methyl ketones, aromatic amines, and products are obtained in moderate to high yields (up to 96%) with high stereo selectivities (>99% e.e.). The authors claim the process to be fairly general, and the catalyst system was recyclable at least thrice without significant loss of efficiency Citation59. Same asymmetric synthesis was carried out by the Liu group under mild conditions using amide-task-specific ILs (AILs)/L-proline to obtain products in high yields and good enantioselectivities (72–96%, 28–99% ee) Citation60. Barbas et al. observed that Mannich reactions, in the presence of 5 mol% of L-proline, were 4–50 times faster in [bmim]BF4 than in organic solvents. It is of interest to note that both the diastereoselectivities and the enantioselectivities of the reactions were excellent (dr 19:1, 99% ee; ). The catalyst in IL was used over four consecutive reaction cycles with only a slight decrease in yields and constant enantioselectivity was observed. The authors also noted a poor performance of hydroxyacetone in Mannich reactions in ILs Citation61. A catalytic amount of RuCl2(PPh3)3, a cross-coupling of 3-buten-2-ol with aldehydes and imines was developed via a tandem olefin migration aldol Mannich reaction in bmim[PF6] proposed by Yang. With In(OAc)3 as a co-catalyst, α-vinylbenzyl alcohol and aldehydes underwent similar coupling reactions. Compared with aqueous and other organic solvents, complementary diastereoselectivity was observed with IL as the solvent. The IL/catalyst system could be reused at least five times without any significant loss of activity Citation62. The Chen group developed another highly asymmetric Mannich-type reaction catalyzed by InCl3 or In(OTf)3 using [bmim][BF4 -] ILs; the reactions proceeded smoothly at room temperature and gave high diastereoselectivities and yields. Contrary to the reaction carried out in water, even enolizable aldimines could efficiently be used in this system Citation63.
Reformatsky reaction
The Reformatsky reaction Citation64 involves condensation of aldehydes (or ketones), with α-halo esters, using suitably activated metallic zinc to obtain β-hydroxy-esters () and this was discovered way back in 1887 by Sergei Nikolaevich Reformatsky Citation65–69.
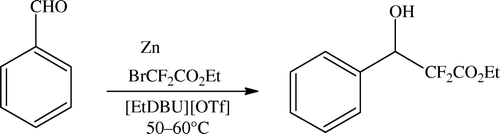
The organozinc reagent is called a “Reformatsky enolate” and is prepared by treating an alpha-halo ester with zinc dust which is less reactive than lithium enolates or Grignard reagents. Full discussion is not relevant here because now several variants and several activators are available to chemists. As per theme of the paper we describe here the role of ILs in this reaction. Kitazume and Kasai reported use of ILs as green alternatives for the production and use of organozinc reagents; see .
Here also, yields are as good as with conventional solvents, product extraction is straightforward, and no VOC problem is encountered Citation70. In the repost also, IL ([EtDBu][OTf]) is used at 50–60°C and the reaction smoothly proceeded to give 76% yield. Moreover, in this reaction system, the yield was increased to 93% when increasing the molar ration of PhCHO:BrCF2CO2Et (1:3).
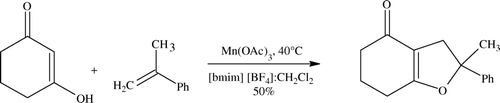
Another variant reported here employs manganese (see ) and the reaction is claimed to be fairly general and facile. IL used is 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]) Citation71.
Streacker reaction
Historically, alpha amino nitriles is the oldest multi-component reaction reported by Streacker, which employs aldehydes, amines, and sodium cyanide/potassium cyanide to afford α-amino nitriles Citation72. This involves addition of cyanide to C = N bond, a common strategy to obtain α-amino nitriles, which serve as important synthons in organic chemistry for preparation of a variety of heterocycles. These nitriles can be conveniently converted into a variety of amino acids Citation73 and several nitrogen heterocycles like thiadiazoles, Citation74 imidazoles, Citation75 and other biologically significant compounds such as saframycin A Citation76 .
Invariably on mixing aldehyde and amine, Schiff's bases are produced which are used in situ and TMSCN addition occurs. Evidently this process requires polarization of C = N bond to facilitate nucleophilic attack of cyanide from TMSCN and is achieved by using suitable Lewis acid and other additives; see Citation77–81. The present authors also tried to replace the catalysts used with greener ones, i.e. milder ones in this reaction Citation82 Citation83. Certainly, the authors were successful in using non-waste producing additives.
Mojtahedi et al. demonstrated the first efficient and environmentally friendly use of 1-butyl-3-methyl-1H-imidazolium perchlorate [bmim][CLO4] IL as the catalytic recyclable media for three-component conversion of aldehydes, amines, and TMSCN to α-aminonitriles at room temperature in excellent yields in one-pot procedure with short reaction times, and IL used was reusable several times Citation84. Another IL 1-butyl-3-methylimidazolium tetrafluoroborate [bmim]BF4 is reported for this process Citation85.
Barbier reaction
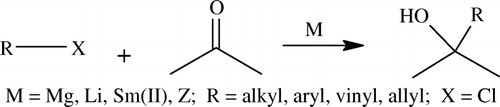
This reaction was discovered by P. Barbier in 1899 Citation86. Classically, it is a reaction between an alkyl halide and a carbonyl group in the presence of suitably activated metal zinc; later on several organic halides and metals were shown to participate in this reaction, such as aluminum, zinc, indium, tin, etc. Usually this reaction is restricted to allyl halides and in situ allylic metal systems are formed which add on to carbonyl group to afford secondary or tertiary alcohols (see ).
From time to time, improvements have been made to make this reaction also environmentally benign and here also the present authors have made some contributions using metals like tantalum and bismuth, cadmium Citation87 Citation88. The introduction of ILs in organic synthesis also prompted researchers to use these in this reaction as well. So, Zhao et al. reported Barbier allylation in one-pot manner using (un)substituted benzaldehydes, allylbromide, and phenols in IL (BuPyCl/SnCl2•2H2O) to directly synthesize 4-(2-hydroxyphenyl)-4-[(un)substituted phenyl]but-1-ene which finally led to the synthesis of 4-(substituted phenyl)-chromans via intramolecular cyclization reactions. This IL was recycled more than four times and it does not affect the yields of products Citation89. Same group also synthesized 4-arylchromans via Barbier allylation through intramolecular hydroalkoxylation of aromatic aldehydes, allylbromides, and phenols in an IL (BPyX-SnCl2·2H2O). They represent intramolecular hydroalkoxylation of 4-aryl-4-(2-hydroxylphenyl)-but-1-enes can be promoted using the Lewis acid ZnCl2 in an IL Citation90.
Pechmann reaction
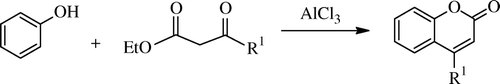
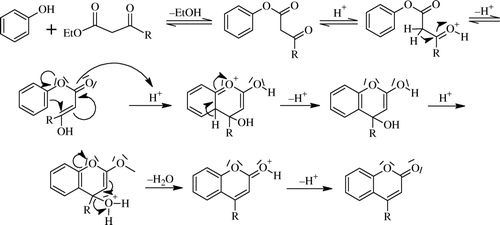
Classically, the Pechmann reaction Citation91 refers to the condensation of β-ketoesters with phenols in the presence of excess of acid catalysts to produce 4-substituted heterocyclic compounds i.e. coumarins, involving tandem hydroxyalkylation, transesterification, and dehydration. Esterification/transesterification followed by attacking the activated carbonyl ortho to the oxygen to generate the new ring via dehydration, as in the case of aldol condensation Citation92 Citation93. A plausible mechanism is also shown here ( and ).
Mechanism of Pechmann reaction
Evidently, the Lewis acids used here are very strong and several others of the same type are used. So, for greener process several mild procedures are reported including our own Citation94. As usual ILs here also had prominent place and were used in this reaction (see ).
1-Butyl-3-methylimidazolium chloroaluminate, [bmim]Cl·2AlCl3 IL was the first IL () which was used as an alternative to conventional acid catalysts in the Pechmann condensation of phenols with ethyl acetoacetate leading to the formation of coumarin derivatives by Potdar Citation95. Similar chloroaluminate IL was used by Khandekar and Khadilkar for the synthesis of substituted coumarins to obtain coumarins in good yields, and they employed 1-Butylpyridinium chloroaluminate IL as a solvent cum catalyst for this condensation Citation96. But these chloroaluminated ILs are very sensitive to hydrolysis; traces of water can change the composition of the melt and the concentration of protons. As a result, it is difficult to accurately control the acidity of these ILs. Another disadvantage with these is that they cannot be stored for a long time and should be prepared freshly. So these problems enthuses chemists to search new green ILs solvents as well as catalysts. Some efforts are discussed in the following paragraphs. An efficient and easy method for preparation of (1-butyl-3-methyl-imidazolium hydrogen sulphate) IL using sodium bisulphate in place of concentrated sulphuric acid by microwave irradiation was developed by Singh, and this acidic room temperature IL has been exploited for the synthesis of coumarins under microwave irradiation and solvent-less conditions reducing time and increasing yields Citation97. Another four non-chloroaluminate acidic ILs have been used by Gu et al. as catalysts for this Pechmann condensations of phenols under solvent-free conditions. These were SO3H-functionalized trifluoromethane sulfonate imidazolium IL and they proved to be the most effective catalyst Citation98. Other variation was carried out using 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim]PF6) IL by Potdar and his group Citation99. Similarly, several ILs are reported, claiming one advantage or the other; these are 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4) and 1-butyl-3-methylimidazolium hexafluorophosphate([bmim]PF6) Citation100, 1-butyl-3-methylimidazolium chloride with NbCl5 Citation101 , anhydrous FeCl3 with ILs [MoeMIm][Tf2N] and [BMIm][Tf2N] Citation102 , and N,N,N-trimethyl-N-propanesulfonic acid ammonium hydrogen sulfate [TMPSA][HSO4] Citation103.
Henry reaction
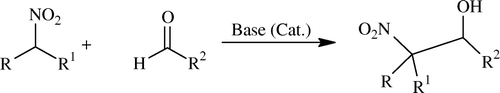
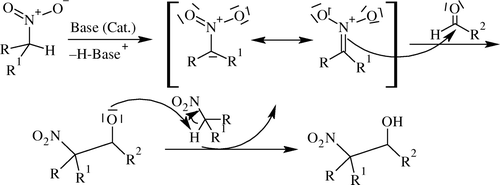
Henry reaction Citation104 is a base-catalyzed carbon–carbon bond-forming reaction Citation105–107 between nitroalkanes and aldehydes or ketones like aldol condensation and also referred to as the nitroaldol reaction ( and ). If acidic protons are available (i.e. when R = H), the products tend to eliminate water to give nitroalkenes. Therefore, only small amounts of base should be used if the isolation of the β-hydroxy nitro-compounds is desired.
Base-catalyzed nitroaldol reaction is associated with various competitive reactions Citation108–112 such as aldol condensation, Cannizzaro reaction, Tishchenko reaction, and Nef-type reaction.
Mechanism of the Henry reaction
Researchers in pursuit of green processes and ILs being attractive used these in this well-known reaction also. Qian et al. Citation113 were the first to employ these in this reaction and they obtained β-Nitroalkanols in good yields, employing 1-ethyl-3-methylimidazolium tetrafluoroborate in this condensation at room temperature with DBU. Another report was published using 1,1,3,3-tetramethyl guanidine (TMG)-based IL by Jiang group. In this protocol, the catalyst could be recycled more than 15 times. This TMG-based IL is shown to be fairly general and is applicable to aliphatic carbonyls Citation114. Another protocol was disclosed by Khan in this reaction at room temperature IL [bmim]BF4 using Na2CO3 as base. The 2-nitroalcohols have been obtained in good yields, and the IL was reusable. However, the diastereoselectivity of the reaction is not very high. When benzaldehyde is used it gives a 35:65 mixture of syn:antinitroalcohols and in case of nitrobenzaldehyde 44:56 of syn:anti nitroalcohols were obtained Citation115. Kumar and Pawar accelerated the Henry reactions in chloroaluminate room temperature ILs. The choloroaluminates with higher compositions of organic species of the chloroaluminates prove to be more efficient rate promoters than the ones with lower organic species in catalyzing Henry reactions, involving both aliphatic and aromatic carbonyl compounds. The ILs can be recycled five times to offer good yields. It should be pointed out that the IL used by Jiang et al. and others are better than this chloroaluminate IL with regard to its recyclability, as they noted a decrease in the yields after five cycles, while their IL was active up to 15 cycles Citation116. Chiral guanidinium chloride salts were synthesized by Wu et al. from 1,3-dimethyl-2-imidazolidinone and (S)-1-phenylethylamine; these ILs are likely to be better potential catalysts for asymmetric Henry reaction Citation117. Burguete and co-workers reported the preparation of new solid base catalysts with suitable mechanical stability for their application for continuous-flow processes. These catalytic systems are based on basic anions immobilized by metathesis onto supported IL-like phases (SILLPs) and have been applied for batch and continuous nitroaldol reaction. This approach synergically combines the advantages of supported ILs as a “supported liquid solvent”, those of an immobilized base as a “green” catalyst, and those of solvent-free reactions with the advantages of a continuous flow process, easy product separation, and catalyst reuse Citation118. Chinese chemists catalyzed Henry reaction using basic IL 1-methyl-3-butylimidazolium hydroxide ([bmIm]OH) with good yields in short time at room temperature. This strategy is general and works over a broad range of aldehydes. The recovered [bmIm]OH can be recycled with consistent activity Citation119. Yadav and Rai developed a convenient three-component coupling reaction of nitromethane, aromatic aldehydes, and trimethylsilyl cyanide (TMSCN) or ammonium thiocyanate for an expeditious synthesis of b-nitrocarbonitriles or b-nitrothiocyanates, respectively, via C–C and C–S bond-forming reactions. The synthetic protocol strategically involves a one-pot sequential Henry reaction and a Michael addition efficiently promoted by the same IL [bmim]OH. The main advantages of the present approach include use of inexpensive simple substrates and an IL as an efficient reaction promoter for the mild synthesis in a one-pot procedure Citation120. Blay et al. discussed enantioselective Henry (nitroaldol) reaction between nitromethane and an aromatic aldehyde, and this was successfully catalyzed by copper complexes of chiral iminopyridine, prepared from camphorsulfonic acid. High yield and good enantioselectivity were achieved Citation121. Henry reaction of benzaldehyde with nitromethane using methoxyl propylamine acetate IL as catalyst is investigated by Wang. Effects of experimental conditions on Henry reaction are studied. Main factors included molar ratio between benzaldehyde and nitromethane, amount of ILs, reaction time, and reaction temperature. Methoxyl propylamine acetate IL is an effective catalyst for Henry reactions under solvent-free conditions. The product separated easily with high yields. Catalyst recycled readily and reused to produce almost identical results Citation122.
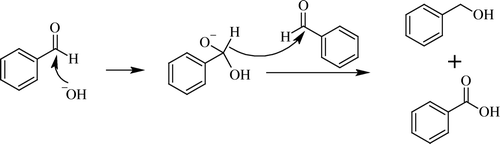
Cannizzaro reaction
This is also named after its discoverer S. Cannizzaro Citation123 in 1853. Classically, this reaction includes the use of caustic alkali, and one molecule of aldehyde is oxidized to acid and the other is reduced to corresponding alcohol Citation124–127 (). In this reaction normally employed bases were strong caustic alkalies like NaOH and KOH etc. In recent years, as in other reactions, development of green processes was attempted, and milder alkalis and solvent-free reactions were reported. In a typical case under solvent-free conditions, KOH pellets were grinded with benzaldehyde to obtain the expected acid and alcohol Citation128. Here, present authors used NaOH under solvent-free condition which effectively catalyzed the reaction in the cross version, i.e. cross-Cannizzaro reaction employing aromatic aldehydes with paraformaldehyde also this reaction could be conducted to obtain oxidized and reduced products in excellent yields when basic alumina is used in place of caustic alkalis Citation129
In pursuit of further greener versions organo-catalyst has been used employing LiBr along with triethyl amine Citation130 Also, another exciting organo-catalyst based on guanidines is reported in recent years Citation131.
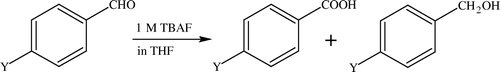
Regarding the utility of ILs in this reaction, there does not seem to be extensive literature available, rather it is scanty and indirectly ILs is used coupled with TBAF/[bmim][PF6] as shown below Citation132 ().
To conclude this account it is worthwhile to state that the entire set of synthetic reactions of organic chemistry is attracting much attention from chemists for developing greener reactions; and in chase of this ILs are abundantly used as discussed and some of the ILs are used along with other additives and even in presence of water. It is certainly hoped that all these efforts of reducing waste, avoiding VOCs would certainly help in sustaining the environment. Authors have tried to bring in this article the recent developments in these discussed named reaction and if any important work of any author is left out, it is sincerely regretted.
Acknowledgements
Authors wish to thank Council of Scientific and Industrial Research (CSIR), New Delhi, India, and Indian National Science Academy (INSA), New Delhi, India, for financial support for this research project.
References
- Holder , J. and Lee , M. 2007 . Environmental protection, law and policy: Text and materials , Cambridge University Press : Cambridge .
- http://www.epa.gov/greenchemistry/pubs/pgcc/presgcc.html
- http://www.rsc.org/Publishing/Journals/gc/News/Greenasp
- http://www.gscn.net/aboutE/index.html
- http://www.chemsoc.org/networks/gcn/awards.htm
- http://nobelprize.org/nobel_prizes/chemistry/laureates/2005/press.html
- Andrade , C.K.Z. ; Alves , L.M. Curr. Org. Chem . 2005 , 9 , 195 – 218 .
- Ohno , H. Bull. Chem. Soc. Japan . 2006 , 79 , 1665 – 1680 .
- Figueras , F. ; Kantam , M.L. ; Choudary , B.M. Curr. Org. Chem . 2006 , 10 , 1627 – 1637 .
- Anastas , P.T. and Warner , J.C. 1998 . Green Chemistry: Theory and Practice , Oxford : Oxford University Press .
- Anastas , P.T. and Williamson , T.C. 1998 . Green Chemistry: Frontiers in Benign Chemical Synthesis and Processes , New York : Oxford University Press .
- Rothenberg , G. ; Downie , A.P. ; Raston , C.L. ; Scott , J.L. J. Am. Chem. Soc . 2001 , 123 , 8701 – 8708 .
- Toda , F. ; Tanaka , K. ; Sekikawa , A. J. Chem. Soc. Chem. Commun . 1987 , 4 , 279 – 280 .
- Tanaka , K. ; Toda , F. Chem. Rev . 2000 , 100 , 1025 – 1074 .
- Lubineau , A. ; Auge , J. ; Queneau , Y. Synthesis 1994 , 8 , 741 – 760 .
- Zhou , C.L. ; Zhou , Y.Q. ; Wang , Z.Y. Chin. Chem. Lett . 2003 , 14 , 355 – 358 .
- Mikami , K. 2005 . Green Reaction Media in Organic Synthesis , Oxford : Blackwell publishing Ltd .
- Adams , D.J. , Dyson , P.J. and Tavener , S.J. 2004 . Chemistry in alternative reaction media , England : John Wiley & Sons Ltd .
- E. Adv . Heterocycl. Chem . 2006 , 90 , 1 – 123 .
- Ashry , E.S.H. ; Ramadan , E. ; Kassem , A.A. ; Hagar , M. Adv. Heterocycl. Chem . 2005 , 88 , 1 – 110 .
- http://www.aspbs.com/jnn/;http://pubs.acs.org/journal/nalefd?cookieSet=1; ; http://www.sciencedirect.com/science/journal/17480132 .
- Suresh; Sandhu , J.S. , Green Chem. Lett. Rev . 2011 , In Press .
- Mannich , C. and Krösche , W. 1912 . Arch. Pharmazie 647 – 667 .
- Kobayashi , S. and Ishitani , H. 1999 . Chem. Rev 1069 – 1094 .
- Cordova , A. Acc. 2004 . Chem. Res 102 – 112 .
- Leadbeater , N.E. , Torenius , H.M. and Tye , H. 2003 . Mol. Divers 135 – 144 .
- Aberg , V. ; Almstedt , H. ; Westermark , A. ; Almqvist , F. J. Org. Chem . 2004 , 69 , 7830 – 7835 .
- Hu , D.Y. , Song , B.A. , Zhang , G.P. , Yang , S. , He , W. , Wu , Y.L. , Hong , Y.P. , Jin , L. H. and Liu , G. 2005 . Chin J.Org. Chem 854 – 858 .
- Loh , T.P. , Liung , S. and Wei , L.L. 2000 . Tetrahedr. Lett 323 – 326 .
- Komoto , I. and Kobayashi , S. 2001 . Chem. Commun 1842 – 1843 .
- Yang , Y.Y. , Shou , W.G. and Wang , Y.G. 2006 . Tetrahedron 10079 – 100861 .
- Takahashi , E. , Fujisawa , H. and Mukaiyama , T. 2004 . Chem. Lett 936 – 937 .
- Yi , L. , Lei , H.S. , Zou , J.H. and Xu , X.J. 1991 . Synthesis 717 – 718 .
- Chen , H.L. , Zou , J.H. and Chin , J. 1999 . Southwest Norm Univ 644 – 647 .
- Iimura , S. , Nobutou , D. , Manable , K. and Kobayashi , S. 2003 . Chem. Commun 1644 – 1645 .
- Wang , L. , Han , J. , Sheng , J. , Tian , H. and Fan , Z. 2005 . Catal. Commun 201 – 204 .
- Yi , W.B. ; Cai , C.J. Fluorine Chem . 2006 , 127 , 1515 – 1521 .
- Rodriguez , B. and Bolm , C J. 2006 . Org. Chem 2888 – 2891 .
- List , B. , Pojarliev , P. , Biller , W.T. and Martin , H.J J. 2002 . Am. Chem. Soc 827 – 833 .
- Ibrahem , I. , Casas , J. and Cordova , A. 2004 . Angew Chem. Int. Ed 6528 – 6531 .
- Gadhwal , S. , Baruah , M. , Prajapati , D. and Sandhu , J.S. 2000 . Synlett 341 – 342 .
- Fang , D. , Gong , K. , Zhang , D.-Z. and Liu , Z.-l. 2009 . Monatsh. Fur. Chemie 1 – 5 .
- Fang , D. ; Cao , S.-T. ; Fei , Z.-H. .; Liu , Z.-L. , Hann. Caili./Chin. J. Energetic Mater . 2009 , 17 , 404 – 407
- Dong , F. ; Zhenghao , F. ; Zuliang , L. , Cata. Commun . 2009 , 10 , 1267 – 1270 .
- Gong , K. ; Fang , D. ; Shi Q.-R. ; Liu , Z.-L. , Hann. Caili./Chin. J. Energetic Mater . 2008 , 16 , 121 – 124 .
- Gong , K. ; Fang , D. ; Wang , H.-L. ; Liu , Z.-L. , Monatsh. Fur. Chem . 2007 , 138 , 1195 – 1198 .
- Fang , D. ; Liu , Z. , Nan. Hangk. Hangti. Dax. Xue. /J. Nanji.Uni. Aeronautics and Astronautics . 2007 , 39 , 540 – 543 .
- Dong , F. ; Jun , L. ; Xin-Li , Z. ; Zu-Liang , L. , Cata. Lett . 2007 , 116 , 76 – 80 .
- Vale , J.A. , Zanchetta , D.F. , Moran , P.J.S. and Rodrigues , J.A.R. 2009 . Synlett 75 – 78 .
- Rasalkar , M.S. ; Bhilare , S.V. ; Deorukhkar , A.R. ; Darvatkar , N.B. ; Salunkhe , M.M. , Cana. J. Chem . 2007 , 85 , 77 – 80
- Sahoo , S. ; Joseph , T. ; Halligudi , S.B. , J. Mole. Cata. A: Chemical . 2006 , 244 , 179 – 182 .
- Li , J. ; Peng , Y. ; Song , G. , Cata. Lett . 2005 , 102 , 159 – 162 .
- Akiyama , T. ; Suzuki , A. ; Fuchibe , K. , Synlett 2005 , 6 , 1024 – 1026 .
- Liu , B. ; Xu , D. ; Luo , S. ; Zhang , F. ; Xu , Z. , Huag. Xueb./J. Chem. Ind. Engg.(China) . 2004 , 55 , 2043 – 2046 .
- Gao , H. ; Han , B. ; Li , J. ; Jiang , T. ; Liu , Z. ; Wu , W. ; Chang , Y. ; Zhang , J. , Synth. Commun . 2004 , 34 , 3083 – 3089 .
- Zhao , G. ; Jiang , T. ; Gao , H. ; Han , B. ; Huang , J. ; Sun , D. , Green Chem . 2004 , 6 , 75 – 77 .
- Yang , X.-F. ; Wang , M. ; Varma , R.S. ; Li , C.-J. , Org. Lett . 2003 , 5 , 657 – 660 .
- Lee , S.-G. ; Park , J.H. , Bull. Kor. Chem. Soc . 2002 , 23 , 1367 – 1368 .
- Liu , B.-Y. ; Zhao , D.-S. ; Xu , D.-Q. ; Xu , Z.-Y. , Chem. Res. Chin. Uni . 2007 , 23 , 163 – 168 .
- Liu , B. ; Xu , D. ; Dong , J. ; Yang , H. ; Zhao , D. ; Luo , S. ; Xu , Z. , Synth. Commun . 2007 , 37 , 3003 – 3010 .
- Chowdari , N.S. ; Ramachary , D.B. ; Barbas , C.F. , Synlett 2003 , 12 , 1906 – 1909 .
- Yang , X.-F. ; Wang , M. ; Varma , R.S. ; Li-J , C. , J. Mole. Cata. A: Chem . 2004 , 214 , 147 – 154 .
- Chen , S.-L. ; Ji , S.-J. ; Loh , T.-P. , Tetrahed. Lett . 2003 , 44 , 2405 – 2408 .
- Ocampo , R. ; Dolbier W.R. Jr. , Tetrahedron 2004 , 60 , 9325 – 9374 .
- Reformatsky , S. , Ber . 1887 , 20 , 1210 1211
- Diaper , D.G.M. and Kukis , A. 1959 . Chem. Rev 89
- Rathke , M.W. 1975 . Org. React 423 – 458 .
- Dekker , J. ; Budzelaar , P.H.M. ; Boersma , J. ; Van der Kerk , G.J.M. ; Spek , A.J. , Organometallics 1984 , 9 , 1403 – 1407 .
- Miki , S. , Nakamoto , K. , Kawakami , J. , Handa , S. and Nuwa , S. 2008 . Synthesis 409 – 412 .
- Kitazume , T. ; Kasai , K. , Green Chem . 2001 , 3 , 30 – 32 .
- Bar , G. , Parsons , A.F. and Thomas , C.B. 2001 . Chem. Commun 1350 – 1351 .
- Strecker , A. 1850 . Ann. Chem . Pharm , 75 : 27 – 45 .
- March , J. , Advanced Organic Chemistry , 4th ed . Wiley, New York, p. 965, 1995, pp. 965; Shafran, Y.M.; Bakulev, V.S.; Mokrushin, V.S. Russ. Chem. Rev. 1989 , 58 , 148 – 162 .
- Weinstock , L.M. ; Davis , P. ; Handelsman , B. ; Tull , R. , J. Org. Chem . 1967 , 32 , 2823 – 2829 .
- Mantier , W.L. ; Owens , D.A. ; Comer , W.T. ; Deitchman , D. ; Ferguson , H.C. ; Seidehamel , R.J. ; Young , J.R. , J. Med. Chem . 1973 , 16 , 901 – 908 .
- Duthaler , R.O. 1994 . Tetrahedron , 50 : 1539 – 1650 .
- Chen , W-Y. and Lu , J. 2005 . Synlett 2293 – 2296 .
- Heydari , A. , Khaksar , S. , Pourayoubi , M. and Mahjoub , A.R. 2007 . Tetrahed Lett”. , 48 : 4059 – 4060 .
- Mulzer , J. , Meier , A. , Buschmann , J. and Luger , P. 1996 . Synthesis 123 – 131 .
- De , S.K. , Synth. Commun . 2005 , 35 , 653; De, S.K., Synth. Commun. 2005, 35 , 1577 – 1582 .
- Narasimhulu , M. , Reddy , T.S. , Mahesh , K.C. , Reddy , S.M. , Reddy , A.V. and Venkateswarlu , Y. 2007 . J. Mol. Catal A.: Chem , 264 : 288 – 292 .
- Saini , A. ; Suresh; Sandhu , J.S. , Synth. commun . 2008 , 38 , 3665 – 3661 .
- Suresh; Saini , A. and Sandhu , J.S. 2009 . J. Ind. Chem. Soc. , 86 : 261 – 264 .
- Mojtahedi , M.M. ; Abaee , M.S. ; Abbasi , H. , J.Irani. Chem. Soc . 2006 , 3 , 93 – 97 .
- Yadav , J.S. ; Reddy , B.V.S. ; Eshwaraiah , B. ; Srinivas , M. ; Vishnumurthy , P. , New J. Chem . 2003 , 27 , 462 – 465 .
- Barbier , P. 1899 . Comp. Rend , 128 : 110 – 111 .
- Bhuyan , P.J. , Prajapati , D. and Sandhu , J.S. 1993 . Tetrahed. Lett 7975 – 7976 .
- Sain , B. , Prajapati , D. and Sandhu , J.S. 1992 . Tetrahed. Lett , 33 : 4795 – 4798 .
- Zhao , X.-L. ; Liu , L. ; Chen , Y.-J. ; Wang , D. , Chin. J. Chem . 2007 , 25 , 1312 – 1322 .
- Zhao , X.-L. ; Liu , L. ; Chen , Y.-J. ; Wang , D. , Synlett 2007 , 9 , 1357 – 1364 .
- Pechmann , H.V. , Ber. der deutsch.Chem. Gesells . 1884 , 17 , 929 – 936 .
- Sethna , S.M. ; Shah , N.M. , Chem. Rev . 1945 , 36 , 1 – 62 .
- Joule , J.A. and Mills , K. 2000 . Heterocyclic Chemistry , 4th edn , Oxford, , UK : Blackwell Science .
- Kumar , S. , Saini , A. and Sandhu , J.S. 2007 . Arkivoc , 15 : 18 – 23 .
- Potdar , M.K. ; Mohile , S.S. ; Salunkhe , M.M. , Tetrahed. Lett . 2001 , 42 , 9285 – 9287 .
- Khandekar , A.C. ; Khadilkar , B.M. , Synlett 2002 , 1 , 152 – 154 .
- Singh , V. ; Kaur , S. ; Sapehiyia , V. ; Singh , J. ; Kad , G.L. , Cata. Commun . 2005 , 6 , 57 – 60 .
- Gu , Y. ; Zhang , J. ; Duan , Z. ; Deng , Y. , Adv. Synth. Cata . 2005 , 347 , 512 – 516 .
- Potdar , M.K. ; Rasalkar , M.S. ; Mohile , S.S. ; Salunkhe , M.M. , J. Mole. Cata. A Chem . 2005 , 235 , 249 – 252 .
- Zhu , H.P. ; Yang , F. ; Tang , J. ; He , M.Y. , Green Chem . 2003 , 5 , 38 – 39 .
- Soares , V.C.D. ; Alves , M.B. ; Souza , E.R. ; Pinto , I.O. ; Rubim , J.C. ; Andrade , C.K. Z. ; Suarez , P.A.Z. , Int. J. Mole. Sci . 2007 , 8 , 392 – 398 .
- Kumar , V. ; Tomar , S. ; Patel , R. ; Yousaf , A. ; Parmar , V.S. ; Malhotra , S.V. , Synth. Commun . 2008 , 38 , 2646 – 2654 .
- Dong , F. ; Jian , C. ; Kai , G. ; Qunrong , S. ; Zuliang , L. , Cata. Lett . 2008 , 121 , 255 – 259 .
- Henry , L.C.R. , Hebd. Acad. Sci . 1895 , 120 , 1265 – 1268 .
- Luzzio , F.A. , Tetrahedron 2001 , 57 , 915 – 945 .
- Rosini , G. , In Comprehensive Org. Synth .; Trost , B.M. , Pergamon : New York , 1992 , 2 , 321 – 340 .
- Ballini , R. ; Palmieri , A. , Curr. Org. Chem . 2006 , 10 , 2145 – 2147 .
- Hass , H.B. ; Riley , E.F. , Chem. Rev . 1943 , 32 , 373 – 430
- Hwu , J.R. ; Gilbert , B.A. , J. Am. Chem. Soc . 1991 , 113 , 5917 – 5918 .
- Rosini , G. ; Ballini , R. ; Petrini , M. ; Sorrenti , P. , Synthesis 1985 , 5 , 515 – 517 .
- Melot , J.M. ; Texier-Boullet , F. ; Foucaud , A. , Tetrahed. Lett . 1986 , 27 , 493 – 496 .
- Costantino , U. ; Curini , M. ; Marmottini , F. ; Rosati , O. ; Pisani , E. Chem. Lett . 1994 , 12 , 2215 – 2218 .
- Qian , W. ; Ju , F. ; Bao , W. ; Zhang , Y. , J. Chem. Res . 2004 , 2 , 154 – 155 .
- Jiang , T. ; Gao , H. ; Han , B. ; Zhao , G. ; Chang , Y. ; Wu , W. ; Gao , L. ; Yang , G. , Tetrahed. Lett . 2004 , 45 , 2699 – 2701 .
- Khan , F.A. ; Sudheer , Ch. ; Sahu , N. , Synth. Commun . 2005 , 35 , 201 – 207 .
- Kumar , A. ; Pawar , S.S. , J. Mole. Cata. A: Chem . 2005 , 235 , 244 – 248 .
- Wu , N. ; Wu , H.-H. - Y.-W. ; Jiang Chin. J. Org. Chem . 2008 , 28 , 104 – 110 .
- Burguete , M.I. ; Erythropel , H. ; Garcia-Verdugo , E. ; Luis , S.V. ; Sans V. , Green Chem . 2008 , 10 , 401 – 407 .
- Wu , H. ; Zhang F.-R. ; Wan , Y. ; Ye , L. , Lett. in Org. Chem . 2008 , 5 , 209 – 211 .
- Yadav , L.D.S. ; Rai , A. , Tetrahed. Lett . 2009 , 50 , 640 – 643 .
- Climent , E. ; Fernández , I. ; Hernández-Olmos , V. ; Pedro , J.R. , Tetrahed.-Asymmetry . 2007 , 18 , 1603 – 1612 .
- Wang , W.-J. ; Cheng W.-P. ; Shao , L.-L. ; Liu , C.-H. ; Yang , J.-G. , Kine. Catal . 2009 , 50 , 186 – 191 .
- Cannizzaro , S. , Liebigs Ann 1853 , 88 , 129 – 130 .
- March , J. 1992 . Advanced Organic Chemistry , 4th ed , 1233 – 1235 . New York : John Wiley Sons .
- Trost , B.M. ; Fleming , I. , Comprehensive Organic Synthesis ; New York : Pergamon Press , 1991 ; 8 , p 86 .
- Cannizzaro , S. Annuals 1853 , 88 , 129 – 130 .
- Geissman , T.A. Org. React . 1944 , II , 94 – 113 .
- Phonchaiya , S. ; Panijpan , B. ; Rajviroongit , S. ; Wright , T. ; Blanchfield J.T. , J. Chem. Educ . 2009 , 86 , 85 – 86 .
- Thakuria , J.A. ; Baruah , M. ; Sandhu , J.S. , Chem. Lett . 1999 , 28 , 995 – 996 .
- Mojtahedi , M.M. ; Akbarzadeh , E. ; Sharifi , R. ; Abaee , M.S. , Org. lett . 2007 , 9 , I 2791 – 2793 .
- Basavaiah , D. ; Sharada , D.S. ; Veerendhar , A. , Tetrahed. Lett . 2006 , 47 , 5771 – 5774 .
- Chung , K-H. ; Moon , B-C. ; Lim , C.H. ; Kim , J.P. ; Lee , J.H. ; Chi , D.Y. , Bull. Korean Chem. Soc . 2006 , 27 , 1203 – 1205 .