Abstract
An efficient and convenient method has been developed for the synthesis of substituted coumarin derivatives using a Brønsted acidic ionic liquid as catalyst under solvent-free conditions. The catalyst can be reused for six consecutive runs without significant loss of activity.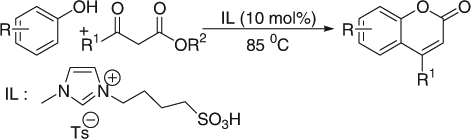
Introduction
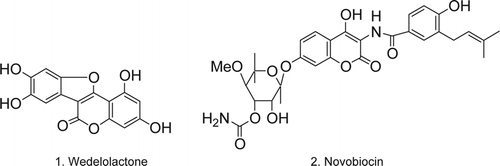
Coumarins are an important class of benzopyrones being the core unit of different natural products and exhibit a spectrum of biological activity Citation1. Natural coumarins, such as calanolides, isolated from Calophyllum genus have shown potent anti-HIV activity Citation2. Wedelolactone 1 is another naturally occurring product that is used as a venomous snake-bite antidote; and Novobiocin 2 () is an antibiotic, which acts as a competitive inhibitor of the bacterial ATP binding gyrase B subunit Citation3. Classical methods for the synthesis of coumarin are Pechmann reaction Citation4, Knoevenagel condensation Citation5, and Wittig reactions Citation6. The Pechmann reaction is one of the simplest and direct methods for the synthesis of coumarins since it proceeds from very simple starting materials, namely, phenols and β-ketoesters in presence of concentrated sulfuric acid. But, due to the direct use of concentrated sulfuric acid, this process causes formation of byproducts and encompasses corrosion problems. Over the years, numerous methods have been developed for the modifications of Pechmann reaction conditions using a variety of reagents Citation7–9. Because of recent efforts toward green chemistry, attempts are being made to replace stoichiometric Brønsted and Lewis acids by non-stoichiometric solid acids, such as montmorillonite clay Citation10, cation-exchanged resin Citation11, and sulfated Ce x Zr1–x O2 Citation12. Ionic liquids have been also employed for this purpose in the Pechmann condensation Citation13–19. Although the reported methods are suitable for certain synthetic conditions, many of these procedures suffered from one or more disadvantages such as harsh reaction conditions, long reaction times, tedious work-up procedure, low selectivity, large amount of catalysts, and also problem of reusability of the catalyst. As coumarin derivatives are extremely important in pharmaceuticals and industry, the development of mild, efficient, and environmentally benign methodology is still desirable.
Results and discussion
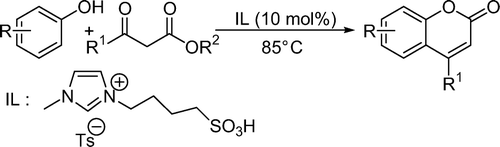
Earlier attempts of coumarin synthesis via Pechmann condensation have been made with chloroaluminate ionic liquids Citation13 Citation14. These ionic liquids are very sensitive to hydrolysis and cannot be stored for a long time. Non-chloroaluminate acidic ionic liquids, which are air and moisture stable, have been developed and applied for acidic reactions. One recent report showed the use of neutral ionic liquid in the presence of POCl3 as catalyst, which is highly toxic and difficult to use Citation15. The application of Brønsted acidic task-specific ionic liquids (TSILs) as catalytic materials is growing continuously in the catalytic field. Combining the useful characteristics of solid acids and mineral acids, TSILs have been synthesized to replace traditional mineral liquid acids, such as hydrochloric acid and sulfuric acid, in chemical reactions. In view of green chemistry, the substitution of harmful liquid acids by reusable TSILs is the most promising catalyst in chemistry. The use of Brønsted acidic TSILs to catalyze organic reactions is an area of ongoing activity and has been successfully used as catalyst for various chemical transformations Citation20–26. In continuation of our interest in ionic liquid-mediated reactions Citation24–28, we have explored the utility of a functionalized ionic liquid, 1-butanesulfonic acid-3-methylimidazolium tosylate, [BSMIm]Ts as a catalyst for the synthesis of coumarin derivatives under solvent-free conditions ().
The experimental procedure is very simple. A mixture of phenolic substrate (2 mmol) and β-ketoester (2 mmol) was heated at 85°C for a certain period of time as required for completion (TLC) in the presence of acidic ionic liquid (10 mol%). The mixture, after being cooled to room temperature was poured into crushed ice (20 g) and stirred for 5–10 min. The solid product was isolated though filtration. A wide range of substituted phenol derivatives and β-ketoesters were examined under the present reaction conditions. The results are summarized in . We studied the reaction of different activated phenols such as resorcinol, phloroglucinol, 3-ethoxyphenol, and α-naphthol with β-ketoester. The reactions were clean and afforded exclusively coumarins in high yields in relatively short times. Ethyl 2-oxocyclopentanecarboxylate also underwent condensation with α-naphthol without any difficulty (entry 7). Acid-sensitive functionalities such as acetyl and tosyl protecting phenols worked very well under the present reaction conditions. This is one of the main advantages of our methodology.
Table 1. Acidic ionic liquid-catalyzed synthesis of coumarins.
Furthermore, in order to show the efficiency and generality of the present protocol we compared some of the results presented here with two recently reported ionic-liquid-based methods Citation17 Citation19 as shown in , which compares reaction time and yields. Thus, it is clear from that the present protocol can act as an effective method with respect to time and yields.
Table 2. Comparison of the present protocol with recently reported methods.
The work-up procedure is so simple and includes filtration of the mixture to separate the product. After isolation of the product from the water mixture, the water layer containing acidic ionic liquid was reused after evaporation of water. We have successfully used the recovered catalyst for the model reaction (entry 1). The recovered catalyst can be recycled successfully without significant loss of activity. After six recycles, the catalyst had a high activity and gave the desired product in good yield (71%, entry 1, ).
Experimental
Melting points were determined on a glass disk with an electrical bath and are uncorrected. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were run in CDCl3 solutions. IR spectra were taken as KBr plates in a Shimazdu 8400S FTIR. Elemental analyses were done by Perkin-Elmer auto analyzer. Ionic liquids were synthesized by following the procedures given in the literature Citation26 Citation29.
Typical procedure for the synthesis of coumarin (entry 9, )
A mixture of α-naphthol (288 mg, 2 mmol) and Ethyl 2-oxocyclopentanecarboxylate (312 mg, 2 mmol) was heated at 85°C for 3 hours (TLC) in the presence of acidic ionic liquid (78 mg, 10 mol%) under solvent-free-conditions. The mixture, after being cooled to room temperature was poured into crushed ice (20 g) and stirred for 5–10 min. The solid product was isolated though filtration and it was re-crystallized from hot ethanol to obtain the pure product (406 mg, 86%). Mp: 213°C; IR: 2958, 2923, 2854, 2340, 2330, 1722 cm−1; 1H NMR ▵: 2.24 (q, 2H, J=7.6 Hz), 2.96 (t, 2H, J=7.6 Hz), 3.13 (t, 2H, J=7.6 Hz), 7.41 (d, 1H, J=8.5 Hz), 7.58–7.63 (m, 2H), 7.66 (d, 1H, J=8.5 Hz), 7.83–7.86 (m, 1H), 8.55–8.57 (m, 1H); 13C NMR ▵: 22.5, 30.5, 32.5, 114.0, 121.0, 122.5, 123.1, 124.1, 127.0, 127.2, 127.7, 128.0, 134.3, 150.9, 157.0, 160.1; Anal. Calcd. (%) for C16H12O2: C 81.34, H 5.12; found: C 81.25, H 5.01.
Conclusion
In conclusion, we have developed a highly efficient methodology for the synthesis of coumarin derivatives in good yields under solvent-free conditions. Operational simplicity, mild reaction conditions, and the reusability of the catalyst are the advantages of the present procedure. In contrast to other acids, storage and handling of this catalyst do not need special precautions, and it can be stored on the benchtop for weeks without losing its activity. We believe that this methodology will be a valuable addition to the existing methods in the field of coumarin derivatives synthesis.
Acknowledgements
Alakananda Hajra is pleased to acknowledge the financial support from DST (Grant No. SR/FTP/CS-107/2006) and Adinath Majee is pleased to acknowledge the financial support from CSIR (Grant No. No. 012251/08/EMR-II).
References
- O’ Kennedy , R. ; Thomas , R.D. Coumarins: Biology, Applications, and Mode of Action ; Wiley & Sons : Chichester , 1997 .
- Kashman , Y. , Gustafson , K.R. , Fuller , R.W. , Cardellina , J.H. , McMahon , J.B. , Currens , M.J. , Buckheit , R.W. , Hughes , S.H. , Cragg , G.M. and Boyd , M.R. 1992 . J. Med. Chem. , 35 : 2735 – 2743 .
- Wu , J. , Lia , Y. and Yang , Z. 2001 . J. Org. Chem. , 66 : 3642 – 3645 .
- Sethna , S.M. and Phadke , R. 1953 . Org. React. , 7 : 1 – 58 .
- Jones , G. 1967 . Org. React. , 15 : 204 – 599 .
- Narsimhan , N.S. ; Mali , R.S. ; Barve , M.V. Synthesis 1979 , 906 – 909 .
- Reddy , Y.T. , Sonar , V.N. , Crooks , P.A. , Dasari , P.K. , Reddy , P.N. and Rajitha , B. 2008 . Synth. Commun. , 38 : 2082 – 2088 .
- Kerri , R.S. , Hosamani , K.M. and Reddy , H.R.S. 2009 . Catal. Lett. , 131 : 321 – 327 .
- Raju , B.C. , Babu , T.H. and Rao , J.M. 2009 . Indian J. Chem. , 48B : 120 – 123 .
- Li , T.S. ; Zhang , Z.H. ; Yang , F. ; Fu , C.G. J. Chem. Res. Synop. 1998 , 38 – 39 .
- John , E.V.O. and Israelstam , S.S. 1961 . J. Org. Chem. , 26 : 240 – 242 .
- Reddy , B.M. , Patil , M.K. and Lakshmimana , P. 2006 . J. Mol. Catal. A: Chem. , 256 : 290 – 294 .
- Amit , K.C. ; Bhushan , K.M. Synlett 2002 , 152 – 154 .
- Potdar , M.K. , Mohile , S.S. and Salunkhe , M.M. 2001 . Tetrahedron Lett. , 42 : 9285 – 9287 .
- Potdar , M.K. , Rasalkar , M.S. , Mohile , S.S. and Salunkhe , M.M. 2005 . J. Mol. Catal. A: Chem. , 235 : 249 – 252 .
- Gu , Y. , Zhang , J. , Duan , Z. and Deng , Y. 2005 . Adv. Synth. Catal. , 347 : 512 – 516 .
- Singh , V. , Kaur , S. , Sapehiyia , V. , Singh , J. and Kad , G.L. 2005 . Catal. Commun. , 6 : 57 – 60 .
- Kumar , V. , Tomar , S. , Papet , R. , Yousaf , A. , Parmar , V.S. and Malhotra , S.V. 2008 . Synth. Commun. , 38 : 2646 – 2654 .
- Soares , V.C.D. , Alves , M.B. , Souza , E.R. , Pinto , I.O. , Rubim , J.C. , Andraze , C.K.Z. and Suarez , P.A.Z. 2007 . Int. J. Mol. Sci. , 8 : 392 – 398 .
- Welton , T. 1999 . Chem. Rev. , 99 : 2071 – 2084 .
- Zhao , H. and Malhotra , S.V. 2002 . Aldrichimica Acta , 35 : 75 – 83 .
- Jain , N. , Kumar , A. , Chauhan , S. and Chauhan , S.M.S. 2005 . Tetrahedron , 61 : 1015 – 1060 .
- Anouti , M. , Caillon-Caravanier , M. , Dridi , Y. , Galiano , H. and Lemordant , D. 2008 . J. Phys. Chem. B , 112 : 13335 – 13343 .
- Ranu , B.C. , Dey , S.S. and Hajra , A. 2003 . Tetrahedron , 59 : 2417 – 2421 .
- Kundu , D. , Debnath , R.K. , Majee , A. and Hajra , A. 2009 . Tetrahedron Lett. , 50 : 6998 – 7000 .
- Das , S. , Rahaman , M. , Kundu , D. , Majee , A. and Hajra , A. 2010 . Can. J. Chem. , 88 : 150 – 154 .
- Kundu , D. , Majee , A. and Hajra , A. 2010 . Catal. Commun. , 11 : 1157 – 1159 .
- Rahman , M. , Majee , A. and Hajra , A. 2010 . J. Heterocycl. Chem. , 47 : 1230 – 1233 .
- Liu , Q. , Janssen , M.H.A. , van Rantwijk , F. and Sheldon , R.A. 2005 . Green Chem. , 7 : 39 – 42 .