Abstract
The three-component condensation reaction of indoles with carbonyl compounds to yield bis(indolyl)methanes has been accomplished with a catalytic amount of iodine (2 mol%) in the presence of sodium dodecylsulfate (SDS) in aqueous solution above its critical micellar concentration (cmc). The surfactant-aided water-compatible Lewis acid catalyst has been found to be efficient for a wide range of carbonyl compounds including aromatic aldehydes bearing electron-releasing and electron-withdrawing groups, α,β-unsaturated aldehydes, heterocyclic aldehydes, cyclic and aromatic ketones with respect to yield, and reaction time. The protocol has been extended to Michael addition of indoles with α,β-unsaturated ketones exclusively yielding 3-substituted indolyl ketones in excellent yields. 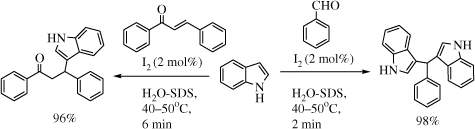
Introduction
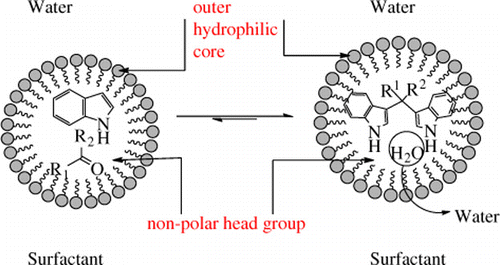
Replacement of volatile organic solvents with nonpolluting alternative reaction media is one of the major aims of green chemistry Citation1. Water is the cheapest, noninflammable and environmentally benign solvent that is involved in life-sustaining biochemical processes in living organisms. It has unique physical and chemical properties, which may be utilized for attaining reactivity and selectivity that cannot be realized in common organic solvents. In addition, it is redundant to dry solvents and reagents in reactions in aqueous medium, thereby vastly reducing consumption of drying agents, and energy. Therefore, there is contemporary paradigm shift to explore organic reactions in aqueous medium Citation2, Citation3 and evaluate various features, most importantly, kinetic and catalytic ones, of organic reactions in aqueous medium. The major hurdle in these efforts is to solubilize organic substrates, particularly nonpolar ones, in water. Utilization of phase-transfer catalysts, water in combination with antihydrophobic additives, reactions on water surface when one of the organic substrates in polar conferring it substantial solubility, and solubilizing organic compounds in aqueous medium in the presence of surfactants are some of the attractive approaches to this end. The micellar solubilization route utilizes inexpensive surfactants as additives that provide heterogeneous microenvironments and, upon self-assembly into micelles, encapsulate otherwise insoluble substrates and catalysts within the lipophilic cores. The concept of surfactant-aided Lewis and Brønsted acid catalysts to mediate carbon–carbon bond formation reactions in water has been developed by Kobayashi et al. Citation4–7 and applied with remarkable success to a host of organic transformations Citation8–14. Development of newer protocols for the synthesis of bis(indolyl)methanes is a topic of broad interest over the years. Diheteroaryl-methane motifs in general and bis(indolyl)methanes in particular are important bioactive metabolites of terrestrial and marine origin Citation15–20. Bis(3′-indolyl)methane itself is a chemopreventive agent from cruciferous plants, and these compounds can act as highly selective fluorescent molecular sensors for Cu+2 ions Citation21 and colon cancer cell inhibitors Citation22–24. The synthesis of these molecules is traditionally based upon a multicomponent condensation of two molecules of indoles as nucleophilic partners with one molecule of a carbonyl compound in the presence of Brønsted and Lewis acids. A good number of methods are documented in the literature that employ various protic and Lewis acid catalysts such as HCl Citation25, HClO4-SiO2 Citation26, KHSO4 Citation27 Citation28, LiClO4 Citation29, Zr(IV) salts Citation30–32, InCl3 Citation33 Citation34, SbCl3 Citation35, CeCl3.7H2O Citation36 Citation37, SmI3 Citation38, lanthanide triflates Citation39–42, La(NO3)3. 6H2O Citation43, and ionic liquids Citation44. However, most of these methods suffer from one or more limitations such as use of expensive catalysts, lack of procedural simplicity such as rigorous exclusion of moisture, toxicity of catalyst. Even when the desired reaction proceeds, trapping of Lewis acids by indole nitrogen necessitates their use in more than stoichiometric amounts Citation45. In continuation of our interest in organic reactions in aqueous micellar medium mediated by water-tolerant Lewis acids Citation46–48, we became interested to evaluate the efficacy of iodine catalyst in water in the presence of anionic surfactant sodium dodecylsulfate (SDS) for the synthesis of bis(indolyl)methanes. Recently, this catalytic combination has been effectively used for the deoximation Citation49, iodohydrin formation Citation50 and, in fact, utilization of iodine as a mild Lewis acid to promote carbon–carbon and carbon–oxygen bond formation reactions has been amply demonstrated in recent times Citation52–56. Dehydration reactions in water are based upon the concept that Lewis acid catalyst and organic substrates with polar heads would form emulsion droplets with a hydrophobic interior, and surfactant molecules would help to concentrate iodonium cation or metal cation on the surface of these droplets. It was surmised that water formed during the condensation of indoles with carbonyl compounds would be excluded out of hydrophobic core of the micelles, thereby favoring the thermodynamic equilibrium toward formation of the desired target molecules (). Previously, 20 mol% of iodine has been employed in acetonitrile Citation57 as also solvent-free conditions Citation58 for the synthesis of bis(indolyl)methanes. We set out to explore iodine as a catalyst in aqueous micellar environment and reveal herein iodine-catalyzed synthesis of bis(indolyl)methanes in water.
Results and discussion
We examined the base-level reactivity of benzaldehyde toward indole at electron-rich C-3 position to afford 1 in the presence of SDS without iodine catalyst (entries 1–2, ).
Table 1. Optimization experiments for the synthesis of bis(indolyl)methane 1.
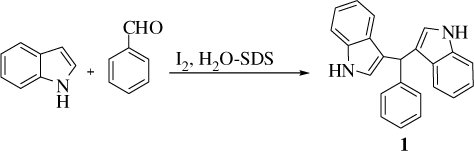
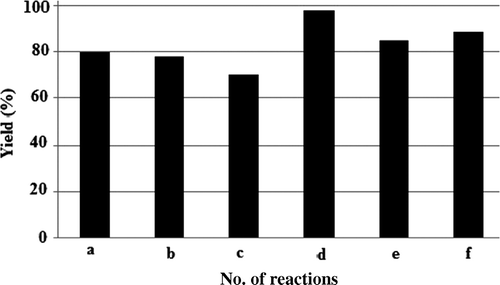
These initial exploratory experiments resulted in the isolation of 1 in moderate yields without iodine in a sluggish reaction at room temperature. To our delight, there was dramatic acceleration in reaction rate upon addition of 2 mol% of iodine in a thoroughly stirred aqueous solution containing 5 cmc of SDS at room temperature (5 min, 78%). The cationic and neutral surfactants, namely, CTAB (cetyltrimethylammonium bromide) and Triton X-100, were less effective with identical catalyst load under comparable conditions. Encouraged by the report that surfactant-aided catalysts could sustain high temperature Citation5 the temperature of the reaction was increased to 40–50°C with further enhancement of reaction rate and yield ().
Figure 1. Variation of yield (iodine used in all cases: 2 mol%, except in “c” where 1 mol% iodine was used) of 1 in I2-catalyzed synthesis under aqueous micellar conditions. a SDS (10 cmc, 5 min, rt); b SDS (5 cmc, 10 min, rt); c SDS (10 cmc, 15 min, rt); d SDS (5 cmc, 40–50°C, 2 min); e CTAB (10 cmc, 40–50oC, 10 min); f Triton X 100 (10 cmc, 40–50°C, 12 min).
The optimized reaction conditions [2 mol% iodine, SDS (5 cmc), 10 mL water mmol-1 of carbonyl compound, 40–50°C] worked remarkably well for a wide range of carbonyl compounds, as shown in .
Table 2. I2-catalyzed synthesisa of bis(indolyl)methanes in aqueous micellar solution.
However, steric congestion around carbonyl carbon impedes the reaction rate substantially as reflected in the longer reaction time required for sterically encumbered aromatic aldehydes and ketones (entries 13–15). Gratifyingly, the current method is superior to existing iodine-based methods in bringing down the catalyst load from 20 to 2 mol% without compromising efficiency and operational simplicity. The major reason for this success is presumably due to activation of iodine catalyst by SDS Citation50 , in addition to its facilitating role in solubilizing substrates and iodine. This protocol was further extended to the chemical space of Michael addition of indoles to α,β-enones, leading to 3-substituted indolyl ketones. These compounds form building blocks of several biologically important indolyl alkaloids such as hepalindole D Citation59 This area has been revisited in recent times Citation35 from green chemistry perspectives and application of I2-SDS-H2O catalytic system is still unprecedented. The results of Michael addition of indoles with several α,β-unsaturated ketones are exhibited in .
Table 3. I2-catalyzed Michael additiona of indole with α,β-unsaturated ketones.
Notably, other electron-poor C = C systems such as methyl acrylate, acrylonitrile, β-nitrostyrene, and p-benzoquinone were resistant to addition under the conditions of the reaction. It is also remarkable that no N-alkylated byproduct was formed in this reaction. Solid-state iodine-catalyzed (5 mol% of iodine) addition of enones to indoles Citation60 is reported to afford Michael adducts in varying yields (45–85%) in 20–30 minutes; however, the process suffers from the limitation of using fairly large amount of volatile halogenated solvent (25 mL of dichloromethane per mmol scale) to bring the product into solution. The current protocol is superior to this method in terms of better yields (76–98%) and avoids the use of any environmentally unacceptable solvent. InCl3 Citation61, Citation62 in 10 mol% catalytic amount is reported to afford Michael addition products in 10–24 h in organic solvents. This is, therefore, a method of choice with the right balance of cost compatibility, short reaction times, and good-to-excellent yields.
Experimental
Typical experimental procedure for synthesis of bis(indolyl)methane 1 from indole and benzaldehyde with iodine catalyst in water containing SDS: Finely pulverized solid iodine (5 mg, 2 mol%) was added to a white emulsion SDS (116 mg, 0.4 mmol, 5 cmc) in water (10 mL) and thoroughly stirred until iodine was dissolved imparting brown color to the resulting mixture. It was warmed to 40–50°C and benzaldehyde (106 mg, 1 mmol) followed by indole (234 mg, 2 mmol) were added when instant reaction occurred. TLC monitoring showed complete disappearance of starting materials within 2 minutes. The reaction was quenched by addition of a 5% solution of Na2S2O3 (3 mL) and then brine. Extraction with ethyl acetate (3×5 mL), washing the combined organic extract with water (2×2 mL), drying (Na2SO4) and removal of solvent under reduced pressure yielded almost pure 1. It was further purified by filtration through a short pad of silica gel (60–120 mesh) using ethyl acetate-hexane (1:4) as eluent to afford 1 (315 mg, 98%), mp 124–126oC [lit. Citation26 125–127oC].
Conclusion
A protocol of synthesis of bis(indolyl)methanes and 3-substituted indolyl ketones has been developed employing 2 mol% of iodine catalyst in aqueous solution in the presence of SDS under warming condition. The surfactant-aided catalytic method in aqueous micellar environment has several key advantageous features such as utilization of inexpensive nontoxic water-tolerant iodine in lower catalyst load compared to that in acetonitrile and under solvent-free conditions, excellent yield and short reaction time.
Acknowledgements
PM is thankful to CSIR, New Delhi, India, for the award of JRF; and SKB thanks University of Kalyani for financial assistance by way of a research fellowship. Facilities provided by DST-FIST and UGC-SAP Grants, Government of India are also acknowledged.
References
- Anastas , P.T. ; Warner , J.C. Green Chem . Oxford : Theory and Practice; Oxford University Press , 2000 .
- Grieco , P.A. Ed. Organic synthesis in water ; London : Blackie Academic and Professional , 1998 .
- Li , C.-J. ; Chang , T.-H. Organic Reactions in Aqueous Media ; New York : John Wiley & Sons , 1997 .
- Kobayashi , S. ; Wakabayashi , T. ; Nagayama , S. ; Oyamada , H. Tetrahedron Lett . 1997 , 38 , 4559 – 4562 .
- Manabe , K. , Mori , Y. , Wakabayashi , T. and Nagayama , S. 2000 . J. Am. Chem Soc , 122 : 7202 – 7207 .
- Manabe , K. ; Kobayashi , S. Chem. Commun . 2000 , 669 670 .
- Manabe , K. , Limura , S. , Sun , X.-M. and Kobayashi , S. 2002 . J. Am. Chem Soc , 124 : 11971 – 11978 .
- Iranpoor , N. , Firouzabadi , H. , Safavi , A. and Shekarriz , M. 2002 . Synth Commun , 32 : 2287 – 2293 .
- Iranpoor , N. , Firouzabadi , H. and Shekarriz , M. 2003 . Org. Biomol Chem. , 1 : 724 – 727 .
- Firouzabadi , H. ; Iranpoor , N. ; Nowrouzi , F. Chem. Commun . 2005 , 789 – 791
- Firouzabadi , H. , Iranpoor , N. and Jafari , A. A. 2005 . Adv. Synth Cat , 347 : 655 – 661 .
- Firouzabadi , H. , Iranpoor , N. and Garzan , A. 2005 . Adv. Synth Cat , 347 : 1925 – 1928 .
- Firouzabadi , H. , Iranpoor , N. and Abbasi , M. 2009 . Adv. Synth Cat , 351 : 755 – 766 .
- Firouzabadi , H. , Iranpoor , N. and Alinezhad , H. 2009 . J. Iran. Chem Soc , 6 : 177 – 186 .
- Sundberg , R.J. 1970 . The Chemistry of Indoles , New York : Academic Press .
- Kam , T.S. In Alkaloids, Chemical and Biological Perspectives ; Pelletier , S.W. ,Ed.; Amsterdam : Pergamon , 1999 , 4 , 429 .
- Irie , T. , Kubushirs , K. , Suzuki , K. , Tsukazaki , K. , Umezawa , K. and Nozawa , S. 1999 . Anticancer Res , 31 : 3061 – 3066 .
- Amino , N. , Ohse , T. , Koyano , T. and Umezawa , K. 1996 . Anticancer Res , 16 : 55 – 59 .
- Bifulco , G. , Bruno , I. , Riccio , R. , Lavayre , J. and Bourdy , G. 1995 . J. Nat Prod , 58 : 1254 – 1260 .
- Morris , S.A. and Anderson , R.J. 1990 . Tetrahedron , 46 : 715 – 720 .
- Martinez , R. , Espinosa , A. , Tarraga , A. and Molina , P. 2008 . Tetrahedron , 64 : 2184 – 2191 .
- Appendino , G. , Cicione , L. and Minassi , A. 2009 . Tetrahedron Lett , 50 : 5559 – 5561 .
- Safe , S. , Papineni , S. and Chintharlapalli , S. 2008 . Cancer Lett , 269 : 326 – 338 .
- Lei , P. , Abdelrahim , M. , Cho , S.D. , Liu , S. , Chintharlapalli , S. and Safe , S. 2008 . Carcinogenesis , 29 : 1139 – 1147 .
- Auria , M. 1991 . Tetrahedron , 47 : 9225 – 9230 .
- Kamble , V.T. , Kadam , K.R. , Joshi , N.S. and Muley , D.B. 2007 . Catal Commun , 8 : 498 – 502 .
- Nagrajan , R. and Perumal , P.T. 2004 . Chem Lett , 33 : 288 – 289 .
- Niknam , K. , Zolfigol , M.A. , Sadabadi , T. and Nejati , A. 2006 . J. Iran. Chem Soc , 3 : 318 – 322 .
- Yadav , J.S. ; Reddy , B.V.S. ; Murthy , C.V.S.R. ; Kumar , G.M. ; Madan , C. Synthesis 2001 , 783 – 787 .
- Nagawade , R.R. and Shinde , D.B. 2006 . Acta. Chim Slov , 53 : 210 – 213 .
- Firouzabadi , H. , Iranpoor , N. , Jafarpour , M. and Ghaderi , A. 2006 . J. Mol. Catal A: Chem , 253 : 249 – 251 .
- Zolfigol , M.A. , Salehi , P. , Shiri , M. and Tanbakouchian , Z. 2007 . Catal . Commun , 8 : 173 – 178 .
- Babu , G. , Sridhar , N. and Perumal , P.T. 2000 . Synth Commun , 30 : 1609 – 1614 .
- Pradhan , P.K. ; Dey , S. ; Giri , V.S. ; Jaisankar , P. Synthesis 2005 , 1779 – 1782 .
- Maiti , G. and Kundu , P. 2007 . Synth Commun , 37 : 2309 – 2316 .
- Bartoli , G. ; Bosco , M. ; Foglia , G. ; Giuliani , A. ; Marcantoni , E. Sambri , L. Synthesis 2004 , 895 – 900 .
- Silveira , C.C. , Mendes , S.R. , Líbero , F.M. , Lenardãob , E.J. and Perinb , G. 2009 . Tetrahedron Lett , 50 : 6060 – 6063 .
- Zou , X. , Wang , X. , Cheng , C. , Kong , L. and Mao , H. 2006 . Tetrahedron Lett , 47 : 3767 – 3771 .
- Nagarajan , R. and Perumal , P.T. 2002 . Tetrahedron , 58 : 1229 – 1232 .
- Mi , X.L. , Luo , S.Z. , He , J.Q. and Cheng , J.P. 2004 . Tetrahedron Lett , 45 : 4567 – 4570 .
- Ma , S. , Yu , S. and Peng , Z. 2005 . Org. Biomol Chem , 3 : 1933 – 1936 .
- Alam , M.M. , Varala , R. and Adapa , S.R. 2003 . Tetrahedron Lett , 44 : 5115 – 5119 .
- Selvam , J.J.P. , Srinivasulu , M. , Suryakiran , N. , Suresh , V. , Reddy , S.M. and Venkateswarlu , Y. 2008 . Synth Commun , 38 : 1760 – 1767 .
- Chakraborty , A.K. , Roy , S.R. , Kumar , D. and Chopra , P. 2000 . Green Chem , 10 : 1111 – 1118 .
- Kobayashi , S. , Araki , M. and Yasuda , M. 1995 . Tetrahedron Lett , 36 : 5773 – 5776 .
- Ganguly , N.C. ; Barik , S.K. Synthesis 2008 , 425 – 428 .
- Ganguly , N.C. ; Barik , S.K. Synthesis 2009 , 1393 – 1399 .
- Ganguly , N.C. , Nayek , S. and Barik , S. K. 2009 . Synth Commun , 39 : 4053 – 4061 .
- Gogoi , P. , Hazarika , P. and Konwar , D. 2005 . J. Org Chem , 70 : 1934 – 1936 .
- Cerritelli , S. ; Chiarini , M. ; Cerichelli , G. ; Capone , M. ; Marsili , M. Eur. J. Org. Chem . 2004 , 623 – 630 .
- Fousteris , M. , Chevrin , C. , Bras , J.L. and Muzart , J. 2006 . Green Chem , 8 : 522 – 523 .
- Chu , C. , Gao , S. , Sastry , M.N.V. and Yao , C. 2005 . Tetrahedron Lett , 46 : 4971 – 4974 .
- Gao , S. , Tzeng , T. , Sastry , M.N.V. , Chu , C. , Liu , J. , Lin , C. and Yao , C. 2006 . Tetrahedron Lett , 47 : 1889 – 1893 .
- Lin , C. , Hsu , J. , Sastry , M.N.V. , Fang , H. , Tu , Z. , Liu , J. and Yao , C. 2005 . Tetrahedron , 61 : 11751 – 11757 .
- Yadav , J.S. ; Reddy , B.V.S. ; Reddy , M.S. Synlett 2003 , 1722 – 1724 .
- Firouzabadi , H. , Iranpoor , N. and Hazarkhani , H. J. 2001 . Org Chem , 66 : 7527 – 7529 .
- Bandgar , B.P. and Shaikh , K.A. 2003 . Tetrahedron Lett , 44 : 1959 – 1961 .
- Ji , S.-J. , Wang , S.-Y. , Zhang , Y. and Loh , T.-P. 2004 . Tetrahedron , 60 : 2051 – 2055 .
- Harrington , P.E. ; Kerr , M.A. Synlett . 1996 , 1047 – 1048 .
- Banik , B.K. , Fernandez , M. and Alvarez , C. 2005 . Tetrahedron Lett , 46 : 2479 – 2482 .
- Cozzi , M.P.G. , Giacomooni , M. , Melchiorre , P. , Selva , S. and Ronchi , A.U. 2002 . J. Org Chem , 67 : 3700 – 3704 .
- Yadav , J.S. ; Abraham , S. ; Reddy , B.V.S. Sabitha , G. Synthesis 2001 , 2165 – 2169 .
- Ramesh , C. ; Ravindranath , N. ; Das , B. J. Chem. Res. Synop . 2003 , 72 – 74 .
- Kundu , P. ; Maiti , G. Indian J. Chem. B : 2008 , 47B , 1402 – 1406 .
- Deb , M.L. ; Bhuyan , P.J. Tetrahedron Lett . 2006 , 47 , 1441 – 1443 .
- Singh , P.R. ; Singh , D.U. ; Samant , S.D. Synth. Commun . 2005 , 35 , 2133 – 2138 .
- Reddy , A.V. ; Ravinder , K. ; Reddy , V.L.N. ; Gound , T.V. ; Ravikanth , V. ; Venkateswarlu , Y. Synth. Commun . 2003 , 33 , 3687 – 3694 .
- Zhang , Z.-H. ; Yin , L. ; Wang , Y.-M. Synthesis 2005 , 1949 – 1954 .
- Nair , V. ; Abhilash , K.G. ; Vidya , N. Org. Lett . 2005 , 7 , 5857 – 5859 .
- Wenkert , E. ; Moeller , P.D.R. ; Piettre , S.R. ; McPhail , A.T. J. Org. Chem . 1988 , 53 , 3170 – 3178 .
- Lin , X.-F. ; Cui , S.-L. ; Wang , Y.-G. Synth. Commun . 2006 , 36 , 3153 – 3160 .
- Teimouri , M.B. ; Mivehchi , H. Synth. Commun . 2005 , 35 , 1835 – 1843 .
- Tabatabaeian , K. ; Mamaghani , M. ; Mahmoodi , N. ; Khorshidi A. Can. J. Chem . 2006 , 84 , 1541 – 1545 .
- Liao , B.-S. ; Chen , J.-T. ; Liu , S.-T. Synthesis 2007 , 3125 – 3128
- Nayak , S.K. Synth. Commun . 2006 , 36 , 1307 – 1315
- Murugan , R. ; Karthikeyan , M. ; Perumal , P.T. ; Reddy , B.S.R. Tetrahedron 2005 , 61 , 12275 – 1228 .
- Li , J.-T. ; Dai , H.-G. ; Xu , W.-Z. ; Li , T.-S. J. Chem. Res. Synop . 2006 , 41 – 42 .
- Portela-Cubillo , F. ; Surgenor , B.A. ; Aitken , R.A. ; Walton , J.C. J. Org. Chem . 2008 , 73 , 8124 – 8127 .