Abstract
An efficient one-pot condensation has been developed for the synthesis of polyhydroquinolines via a four component coupling reaction of aldehyde, dimedone, ethyl acetoacetate, and ammonium acetate under catalyst and solvent-free conditions. Non-hazardous experiment procedure, operational simplicity, mild reaction conditions, and the compatibility with various functional groups represent the advantages of the present method.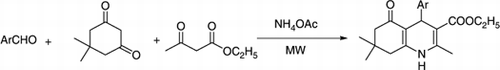
Introduction
1,4-Dihydropyridines exhibit a spectrum of interesting pharmacological properties such as vasodilator, bronchodilator, antiatherosclerotic, antitumor, geroprotective, antidiabetic, calcium channel modulators, and hepatoprotective agents Citation1–4. The synthesis of these privileged heterocyclic compounds has therefore gained special attention. Available methods for synthesis of polyhydroquinoline derivatives are using conventional heating Citation5, refluxing in acetic acid Citation6, ionic liquids Citation7, montmorillonite K10 Citation8 Citation9, organocatalyst Citation10, sulfamic acid Citation11, nickel nanoparticle Citation12, and microwave (MW) irradiation in presence of glycine as a catalyst Citation13. Most of these methods have their own advantages, but also suffer from certain drawbacks such as use of hazardous solvents, long reaction times, low yields, and expensive catalyst. Therefore, there is a need for a simple, efficient, and cost-effective method for the synthesis of polyhydroquinoline derivatives under environmental-friendly conditions.
Results and discussion
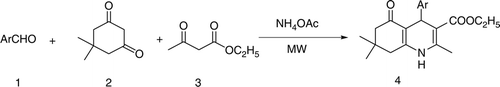
As a part of our ongoing research to provide greener methodology under solvent and catalyst-free conditions Citation14–18, we have found that the four component reaction of aldehyde, dimedone, ethyl acetoacetate, and ammonium acetate under MW irradiation without any solvent and catalyst produced polyhydroquinoline derivatives in high yields within short reaction times ().
In a typical experimental procedure, a mixture of aldehyde (1 mmol), dimedone (1 mmol), ethyl acetoacetate (1 mmol), and ammonium acetate (1.5 mmol) was irradiated in a microwave reactor (CEM, Discover, USA) at 90°C (250 W) for a certain period of time as required for completion (TLC) of the reaction. A wide range of structurally varied aldehydes was subjected under the present reaction conditions to get the corresponding polyhydroquinoline derivatives as summarized in .
Table 1. Synthesis of polyhydroquinoline derivatives.
Several functionalities such as OH, OMe, Cl, Br, F are unaffected under the present reaction conditions. Aromatic aldehydes reacted well to afford the desired products in excellent yields. However, aliphatic aldehydes such as n-butyraldehyde, iso-butyraldehyde and valeraldehyde except cyclohexanecarboxaldehyde (entry 12) produced a mixture of products that are yet to be characterized. The present procedure was also equally effective for methyl acetoacetate (entry 13). In general, the reactions were very fast. The whole operation does not involve any organic solvent. Only ethanol was employed for recrystallization to provide pure compounds.
The efficiency and generality of the present protocol can be realized by comparing some of the results presented here with two recently reported methods, as shown in , which compares reaction time, yields, and reaction conditions. Thus, it is clear from that the present protocol is more practical alternative to reported methodologies with respect to reaction conditions and general applicability.
Table 2. Comparison of the present protocol with recently reported methods.
Experimental
In a typical experimental procedure, benzaldehyde (106 mg, 1 mmol), dimedone (140 mg, 1 mmol), ethyl acetoacetate (130 mg, 1 mmol), and ammonium acetate (115 mg, 1.5 mmol) was irradiated in a microwave reactor (CEM, Discover, USA) at 90°C (250 W) for five minutes as required to complete the reaction (TLC). After completion of reaction, the mixture was poured into crushed ice and stirred for five minutes. The solid separated was filtered under suction (water aspirator), washed with ice-cold water (20 mL), and then recrystallized from hot ethanol to afford analytically pure product (88%, 285 mg) whose identity was established by comparison of its MP and spectroscopic data (IR, 1H NMR) with those reported Citation8.
Conclusion
In conclusion, MW irradiation on a mixture of aldehyde, dimedone, ethyl acetoacetate, and ammonium acetate provides a highly efficient methodology for the synthesis of polyhydroquinoline derivatives in high yields. Operational simplicity, solvent and catalyst-free conditions, and the compatibility with various functional groups are the advantages of the present procedure. We believe that this will present a better and more practical alternative to the existing methodologies for the synthesis of polyhydroquinoline derivatives.
Acknowledgements
A. H. is thankful to Department of Science and Technology, India for financial support (Grant No. SR/S5/GC-05/2010). A. M. is pleased to acknowledge the financial support of CSIR (Grant No. 01(2251)/08/EMR-II).
References
- Bossert , F. , Meyer , H. and Wehinger , E. 1981 . Angew. Chem. Int Ed. , 20 : 762
- Nakayama , H. and Kanaoka , Y. 1996 . Heterocycles , 42 : 901
- Shan , R. , Velazques , C. and Knaus , E.E. 2004 . J. Med Chem. , 47 : 254
- Sabitha , G. ; Reddy , G.S.K.K. ; Reddy , Ch.S. ; Yadav , J.S. Tetrahedron Lett . 2003 , 44 , 4129 .
- Sainani , J.B. and Shah , A.C. 1994 . Indian J. Chem Sect. B. , 33 : 526
- Sufirez , M. , Ochoa , E. , Verdecia , Y. , Verdecia , B. , Moran , L. , Martin , N. , Quinteiro , M. , Seoane , C. , Soto , J.L. , Novoa , H. , Blaton , N. and Peters , O.M. 1999 . Tetrahedron. , 55 : 875
- Ji , S.J. , Jiang , Z.Q. , Lu , J. and Loh , T.P. 2004 . Synlett , 5 : 831
- Song , G. , Wang , B. , Wu , X. , Kang , Y. and Yang , L. 2005 . Synth.Commun. , 35 : 2875
- Maheswara , M. ; Siddaiah , V. ; Damu , G.L.V. ; Rao , C.V. Arkivoc 2006 , ii , 201 .
- Karade , N.K. , Budhewar , V.H. , Shinde , S.V. and Jadhav , W.N. 2007 . Let. Org Chem. , 4 : 16
- Foroughifar , N. , Mobonokhaledi , A. , Fard , M.A.B. , Moghanian , H. and Ebrahimi , S. 2009 . Synth. React. Inorg. Metal-Org. Nano-Met Chem. , 39 : 161
- Sapkal , S.B. , Shelke , K.F. , Shingate , B.B. and Shingare , M.S. 2009 . Tetrahedron Lett. , 50 : 1754
- Singh , S.K. and Singh , K.N. 2010 . J.Heterocyclic Chem. , 47 : 194
- Ranu , B.C. , Hajra , A. and Dey , S.S. 2002 . Org. Process Res Dev. , 6 : 817
- Ranu , B.C. ; Dey , S.S. ; Hajra , A. Arkivoc 2002 , vii , 76 .
- Ranu , B.C. and Hajra , A. 2002 . Green Chem. , 4 : 551
- Rahman , M. ; Roy , A. ; Majee , A. ; Hajra , A. J. Chem. Res . 2009 , 178 .
- Rahman , M. ; Kundu , D. ; Hajra , A. ; Majee , A. Tetrahedron Lett . 2010 , 51 , 2896 .