Abstract
Hydrotrope induced synthesis of 1,8-dioxo-octahydroxanthenes from aromatic aldehydes and dimedone/1,3-cyclohexadione in aqueous medium is reported. The remarkable features of the new procedure are high conversions, shorter reaction times, cleaner reaction profiles, and simple experimental and work-up procedures.
Introduction
Xanthene derivatives are important heterocyclic compounds due to their interesting biological activities such as antiviral Citation1, antibacterial Citation2, anti-inflammatory Citation3, and antinociceptive activities Citation4 possessed by them. They have also been reported for their use as dyes, fluorescent materials for visualization of bio-molecules and laser technologies due to their useful spectroscopic properties Citation5. They are versatile synthons because of inherent reactivity of the inbuilt pyran ring Citation6. The most common strategy for the synthesis of 1,8-dioxo-octahydroxanthenes involves condensation between an aldehyde with dimedone or cyclohexane-1,3-dione. Although the reaction has been investigated using a number of Lewis acid catalysts Citation7–11, there is still scope for improvement especially towards developing a green protocol using highly efficient and reusable catalyst.
Organic solvents are conventionally used in organic synthesis and industrial processes on a large scale. These solvents are often problematic owing to their toxicity and flammability. There is now a realization that more benign chemical synthesis is required, as an integral part of developing sustainable technologies Citation12. Eliminating the use of organic solvents can reduce the generation of waste, which is a requirement of one of the principles of green chemistry. One important aspect of research toward green processes for organic synthesis is the scientific evaluation of potential replacement for volatile organic compounds (VOCs) as solvents and reaction media. Alternative solvents suitable for green chemistry are those that have low toxicity, are inert, easy to degrade, and do not contaminate the product. Alternative media to organic solvents include water Citation13, ionic liquids Citation14 Citation15, supercritical solvents Citation16, polyethylene and polypropylene glycol Citation17, and so on. The term hydrotropes refers to a diverse class of water soluble surface active compounds that enhance the solubilitie's of organic reactants in the aqueous phase at higher concentration. They are capable of increasing the solubility of organic compounds up to 200 times in water. They usually comprise hydrophilic and hydrophobic moieties, with the latter being typically too small to induce micelle formation. Their solubilizing power was recognized as early as 1916 by Neuberg Citation18. The potential use of hydrotropes in industry was stressed in 1946 by McKee Citation19. Aqueous solutions of hydrotropes represent the unique properties of an alternative reaction media for organic synthesis. Besides being cheap, non-toxic, and environment friendly, aqueous hydrotropic solutions possess the other physico-chemical characteristics required to be an alternative reaction media. In our efforts devoted to green chemistry Citation20 Citation21, we recently established the compatibility of aqueous hydrotropic solutions as safer solvents for microwave-assisted reactions Citation22. In continuation of our effort to tap the barely exploited potential of hydrotropes in organic synthesis, we report herein hydrotrope induced synthesis of 1,8-dioxo-octahydroxanthenes in aqueous medium.
Results and discussion
Our investigations began with the scrutiny of an appropriate hydrotrope for the present work. The different hydrotropes such as sodium benzene sulphonate (NaBS), sodium p-xylene sulphonate (NaXS), and sodium p-toluene sulphonate (NaPTSA) were selected for this purpose. Our investigations began with the optimization of aqueous concentration of the selected hydrotropes. After considerable experimentation, we optimized 50% aqueous solution of hydrotropes as solvent since this concentration was suitable for the solubilization of organic compounds in sufficient quantities. Next, we turned our attention to the synthesis of 1,8-dioxo-octahydroxanthenes (). A series of experiments were undertaken in which a mixture of dimedone 1a and benzaldehyde 2a was stirred in 5 mL of 50% of aq. NaBS, NaXS, and NaPTSA in an open air. Preliminary investigations carried out at room temperature did not yield quantitative results. The reaction between 1a and 2a was performed at different temperatures. The best results were obtained when the reaction was carried out at 80 °C (). As evident from the results in , NaPTSA was found to be better alternative as compared to NaBS and NaXS.
Table 1. Screening of various hydrotropes for synthesis of 1,8-dioxo-octahydroxanthenes.a
As excellent results were obtained for NaPTSA, we employed this particular hydrotrope for subsequent studies. To explore the versatility of the protocol, a series of 1,8-dioxo-octahydroxanthenes were synthesized by the reaction of 1a as well as 1,3-cyclohexadione 1b with differently substituted aromatic aldehydes 2b–j in 50% aq. NaPTSA solution at 80 °C The reactions proceeded smoothly in all the cases yielding the corresponding 1,8-dioxo-octahydroxanthenes in good to excellent yields. The results of the reactions are summarized in . With both electron-poor and electron-rich benzaldehydes, the corresponding 1,8-dioxo-octahydroxanthenes were obtained in good to excellent yields. The reaction of the sterically hindered 2-substituted benzaldehydes even gave higher yields highlighting the general applicability of the protocol. The note worthy feature of all the reactions was the easiness of product separation. It can be effected by adding water to the reaction mixture, which precipitates the product that can be isolated merely by filtration. The product obtained after sufficient washings with water was found to be practically pure. The identity of all the products was ascertained on the basis of IR, 1H NMR, 13C NMR, and mass spectroscopy data and is consistent with the literature Citation23 Citation24.
Table 2. Synthesis of 1,8-dioxo-octahydroxanthene in 50% aq. NaPTSA solution.
In order to investigate the role of hydrotrope, the reaction between 1a and 2a was carried out using pure water. The reaction did not gave quantitative yield of corresponding product indicating that the use of NaPTSA is crucial. Aqueous NaPTSA solution was found to be better in terms of cost, handling, remarkable operational simplicity, product isolation, and reaction time.
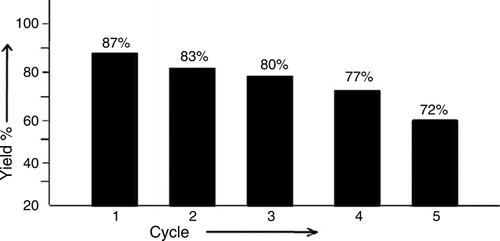
The recovery and reuse of catalysts is highly preferable for a greener process. To check the possibility of the catalyst recycling, the reaction between 1a and 2a was carried out in 50% aq. NaPTSA at 80 °C. After the completion of reaction, the product was separated out from the reaction mixture and the resultant aqueous hydrotropic solution was concentrated and reused in the next cycles without any further purification. As shown in , the aq. NaPTSA solution could be reused at least five times with modest change in the yields of the products.
Experimental
Melting points were determined in an open capillary and are uncorrected. Infrared spectra were recorded on a Perkin-Elmer FTIR spectrometer. The samples were examined as KBr discs ~5% w/w. 1H NMR spectra were recorded on a Bruker Avon 300 MHz spectrometer using DMSO-d6 as solvent and TMS as internal reference. The hydrotropes viz NaBS, NaXS and NaPTSA were synthesized by following procedures reported in the literature Citation25. All other chemicals were obtained from local suppliers and used without further purification.
General procedure for synthesis of 1,8-dioxooctahydroxanthene
A mixture of 1,3-cyclohexadione/dimedone (2 mmol) and aryl aldehyde (1 mmol) in 5 mL of aqueous 50% hydrotropic solution was stirred until a clear solution was formed. The resulting mixture was stirred at 80 °C till the completion of the reaction as monitored by thin layer chromatography. After completion of the reaction, 50 mL of cold water was added to the reaction mixture whereby the crude product precipitated out. The resulting crude product was filtered off, washed with water and recrystallized to afford pure 1,8-dioxo-octahydroxanthenes that were characterized by spectral analysis.
Spectral data of representative compounds
3,3,6,6-Tetramethyl-9-(phenyl)-1,8-dioxo octahydro-oxanthene (Table 2 Entry-a)
IR (KBr): ν = 3039, 2976, 1687, 1669, 1465, 1360, 1201, 1143, 745, 701 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 0.97 (s, 6H), 1.14 (s, 6H), 2.10–2.46 (m, 8H), 4.75 (s, 1H), 7.24–7.46 (m, 5H); 13C NMR (75 MHz, DMSO-d6): 22.75, 29.69, 32.26, 32.61, 41.29, 51.18, 116.07, 126.76, 128.45, 128.80, 144.54, 162.70, 196.76.
9-(4-Hydroxyphenyl)-1,8-dioxo-octahydroxanthene (Table 2 Entry-k)
IR (KBr): ν = 3434, 2951, 1642, 1556, 1373, 1237, 1199, 1185, 775 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 1.78–1.89 (m, 2H), 1.97–2.10 (m, 2H), 2.22–2.30 (m, 4H) 2.48–2.66 (m, 4H), 4.45 (s, 1H), 6.57 (d, 2H), 6.94 (d, 2H), 9.18 (s, 1H); 13C NMR (75 MHz, DMSO-d6): 20.34, 26.87, 30.20, 36.90, 115.11, 116.38, 129.30, 135.50, 156.07, 164.95, 196.83.
9-(2-Hydroxyphenyl)-1,8-dioxo-octahydroxanthene (Table 2 Entry-l)
IR (KBr): ν = 3399, 2923, 16425, 1556, 1372, 1296, 1184, 999, 775 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 1.88 (m, 2H), 1.96 (m, 2H), 2.23 (m, 4H), 2.49 (m, 4H), 5.05 (s, 1H), 6.96 (d, 2H), 7.11 (d, 2H), 10.48 (s, 1H); 13C NMR (75 MHz, DMSO-d6): 20.83, 26.87, 30.03, 36.50, 115.68, 116.40, 135.80, 138.50, 156.40, 165.55, 195.90.
9-(4-Chlorophenyl)-1,8-dioxo-octahydroxanthene (Table 2 Entry-m)
IR (KBr): ν = 3434, 2926, 1668, 1592, 1425, 1359, 1128, 1014, 959, 762 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 1.88 (m, 2H), 1.91 (m, 2H), 2.29 (m, 4H), 2.64 (m, 4H), 4.52 (s, 1H), 7.26 (m, 4H); 13C NMR (75 MHz, DMSO-d6): 26.88, 36.79, 115.50, 128.34, 130.37, 165.46, 196.80.
9-(2-Nitrophenyl)-1,8-dioxo-octahydroxanthene (Table 2 Entry-o)
IR (KBr): ν = 3412, 2925, 1667, 1519, 1456, 1358, 1177, 1013, 962, 786 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 1.86 (m, 2H), 1.95 (m, 2H), 2.27 (m, 4H) 2.64 (m, 4H), 5.40 (s, 1H), 7.35 (d, 2H), 6.94 (d, 2H), 7.56 (m, 1H), 7.77 (d, 1H); 13C NMR (75 MHz, DMSO-d6): 20.20, 26.98, 30.10, 36.82, 114.90, 124.43, 127.71, 131.03, 133.3, 138.9, 155.9, 165.5, 196.8.
Conclusion
A simple, efficient, and green protocol for the synthesis of 1,8-dioxo-octahydroxanthenes in aqueous hydrotropic solution is described. The synthesis has been performed in various hydrotropes. The enhancement in the rate is observed for NaPTSA, which furnishes quantitative yields within 1–2 hours in most of the cases. Furthermore, NaPTSA is easily reused without any appreciable loss in activity.
Acknowledgements
We gratefully acknowledge the financial support from the Department of Science Technology and University Grants Commission for FIST and SAP, respectively.
References
- Lambert , R.W. ; Martin , J.A. ; Merrett , J.H. ; Parkes , K.E.B. ; Thomas , G.J. Chem. Abstr . 1997 , 126 , 212377y, PCT Int. Appl. Wo 9706178, 1997 .
- Hideo , T. Chem. Abstr . 1981 , 95 , 80922; Hideo, T. Jpn. Tokkyo koho J. P. 56005480 1981 .
- Poupelin , J.P. , Saint-Rut , G. , Foussard-Blanpin , O. , Narcisse , G. , Uchida-Ernouf , G. and Lacroix , R. 1978 . Eur. J. Med. Chem. , 13 : 67
- Llama , E.F. , Campo , C.D. , Capo , M. and Anadon , M. 1989 . Eur. J. Med. Chem. , 24 : 391
- Schenkotikhin , Y.M. and Nikolaeva , T.G. 2006 . Chem. Hetrocycl. Comp. , 28 : 42
- Saint-Rut , G. , Hieu , H.T. and Poupelin , J.P. 1975 . Naturwissenschaften. , 62 : 584
- Jin , T.S. ; Zhang , J.S. ; Xio , J.C. ; Wang , A.Q. ; Li , T.S. Synlett 2004 , 5, 886 ; Jin, T.S.; Zhang, J.S.; Xio, J.C.; Wang, A.Q.; Li, T.S. Ultrason. Sonochem. 2006, 13, 220 .
- Khosropour , A.R. ; Khodaei , M.M. ; Moghannian , H. Synlett . 2005 , 6 , 955 .
- Sseyyedhamzeh , M. and Mirzaei , P.A. 2008 . Dyes Pigm. , 78 : 836
- Das , B. , Thirupati , P. , Mahende , I. , Reddy , K. R. , Ravikanth , B. and Nagarapu , L. 2007 . Catal. Commun. , 8 : 535
- Karade , H. ; Sathe , M. ; Kaushik , M. Arkivoc . 2007 , xiii , 252 .
- Anastas , P. ; Warner , J.C. Green Chemistry: Theory and Practice ; Oxford University Press : New York , 1998 .
- Hailes , H.C. 2007 . Org. Process Res. Dev. , 11 : 114
- Holbrey , J.D. ; Seddon , K.R. J. Chem. Soc. Dalton. Trans . 1999 , 13 , 2133 .
- Welton , T. 1999 . Chem. Rev. , 99 : 2071
- Noyori , R. 1999 . Chem. Rev. , 99 : 353
- Chen , J. , Spear , K. , Huddleston , J.G. and Rogers , R.D. 2005 . Green Chem. , 7 : 64
- Neuberg , C. 1916 . Biochem. , Z76 : 107
- McKee , R.H. 1946 . Ind. Eng. Chem. , 38 : 382
- Rashinkar , G. and Salunkhe , R. 2010 . J. Mol. Catal. A: Chem. , 316 : 146
- Rashinkar , G. , Pore , S. and Salunkhe , R. 2009 . Phosphorus Sulfur and Silicon , 184 : 1750
- Rashinkar , G. , Kamble , S. , Kumbhar , A. and Salunkhe , R. 2010 . Transition. Met. Chem. , 35 : 185
- Liu , L.-B. .; Jin , T.-S. ; Han , L.-S ; Li , M. ; Qi , N. ; Li , T.-S . E –J. Chem . 2006 , 3 , 117 .
- Bayat , M. , Imanieh , H. and Hossieni , S.H. 2009 . Chin. J. of Chem. , 27 : 2203
- Furnis , B.S. ; Hannaford , A.J. ; Smith , P.W.G. ; Tatchell , A.R. Vogel's Textbook of Practical Organic Chemistry ; Prentice Hall, Upper Saddle River, NJ, USA , 1996 .
- Tu , S.J. , Gao , Y. , Liu , X.H. , Tang , S.F. and Qiu , X.J. 2001 . Chin. J. Org. Chem. , 21 : 1164
- Jin , T.S. , Zhang , J.S. , Wang , A.Q. and Li , T.S. 2005 . Synth. Commun. , 35 : 2339
- Tu , S.J. , Zhou , J.F. , Lu , Z.S. , Deng , X. , Shi , D.Q. and Wang , S.H. 2002 . Synth. Commun. , 32 : 3063
- Darchive , F. , Balalaie , S. , Chadegani , F. and Salehi , P. 2007 . Synth. Commun. , 37 : 1059
- Horning , E.C. and Horing , M.G. 1946 . J. Org. Chem. , 11 : 95
- Kantevari , S. , Bantu , R. and Nagarapu , L. 2006 . Arkivoc , 16 : 136
- Fan , X. , Hu , X. , Zhang , X. and Wang , J. 2005 . Can. J. Chem. , 83 : 16
- Jung , D.H. , Lee , Y.R. , Kim , S.H. and Lyoo , W.S. 2009 . Bull. Korean Chem. Soc. , 30 : 1989
- Hua , G.P. , Li , T.J. , Zhu , S.L. and Zhang , X.J. 2005 . Chin. J. Org. Chem. , 26 : 716