Abstract
Potassium hydrogen sulfate in the presence of dibenzo-18-crown-6 (DB18C6) turns out to be a very efficient and effective catalyst for the facile synthesis of a wide variety of tri- and tetrasubstituted imidazoles in an aqueous medium at moderate temperature. The idea behind this is that the potassium ion, being trapped in the crown core, frees the hydrogen sulfate anion, which, because of its acidic nature, succeeds in the ready synthesis of the imidazoles.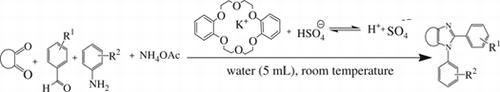
Introduction
The development of new methodologies to produce products of greater structural complexity from readily available simple starting materials with fewer synthetic steps but ensuring high yield under mild reaction conditions is the main challenge for the synthetic organic chemists Citation1 Citation2. Again, because of global environmental legislation on chemical process industries, aqueous environment has been currently receiving considerable attention in organic chemistry Citation(3–5) because water is abundant in nature, has virtually no cost, and is safest among all available solvents, thus leading to environmentally benign chemical processes Citation6.
Synthesis of imidazole ring system and its derivatives occupies an important place in the realm of natural and synthetic organic chemistry as the imidazole moiety, being a part of the side chain in histidine, plays a major role in the biological activity of many peptides and proteins. Several functionalized imidazole derivatives express many pharmacological properties and play important roles in biochemical processes Citation7 . The potency and wide applicability of the imidazole pharmacophore are largely because of its hydrogen-bond donor–acceptor capability as well as because of its high affinity for metals, which are present in many protein active sites Citation8 (e.g. Zn, Fe, Mg). Diverse substituted imidazoles act as inhibitors of p38 MAP kinase Citation9, B-Raf kinase Citation10, glucagon receptors Citation11, plant growth regulators Citation12, therapeutic agents Citation13, antibacterial Citation14, antitumor Citation15, and pesticides Citation16 . Recent advancements in green chemistry and organometallic chemistry expand the utility of imidazoles as ionic liquids Citation(17–19) and N-heterocyclic carbenes Citation20 . Because of their widespread applications in various streams, there is an increasing interest in research on imidazoles.
2,4,5-Trisubstituted imidazoles are usually synthesized by a multicomponent reaction involving benzil (or a substituted benzil), an aldehyde, and ammonium acetate as an ammonia source Citation21, and many catalysts [silica/sulfuric acid Citation22 Citation23, Yb(OTf)3 Citation24, NiCl2·6H2O Citation25, oxalic acid Citation26, iodine Citation27, sulfanilic acid Citation28, tetrabutyl ammonium bromide Citation29, cerium ammonium nitrate Citation30, Zr(acac)4 Citation31, InCl3·3H2O Citation32] as well as different activation modes [thermal activation, microwave irradiation Citation21 Citation33, or ultrasounds Citation30 Citation31] have been used to get the desired imidazoles in high yields. Most of the earlier methodologies are performed either in solution [AcOH Citation21 Citation24, EtOH Citation25 Citation27, EtOH–H2O Citation26 Citation34 Citation35], under solventless conditions Citation22 Citation33 Citation36, or in ionic liquids Citation37 Citation38 . But the synthesis of substituted imidazoles in an aqueous medium is rare. Actually there is only one report in this regard and that too under microwave irradiation at very high temperature (180–210°C) Citation39 . Therefore, room for green methodologies for the rapid construction of substituted imidazoles in an aqueous medium at milder conditions still remains quite open.
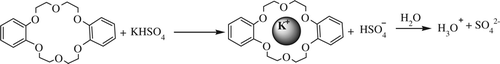
Cyclic polyethers such as 18-crown-6 (18C6) and dibenzo-18-crown-6 (DB18C6) have some unique ability to bind metal cations and small neutral or ionic molecules selectively. Two kinds of binding forces for complexation of cyclic polyethers are well established: ion–dipole interaction and hydrogen bonding Citation40 . Metal ions form complexes with crown ethers through ion–dipole electrostatic interactions, with the oxygen lone pairs being attracted to the cation positive charge () and various organic molecules with acidic hydrogens binding to crown ethers via hydrogen bonding. Now, in the case of a salt, cyclic polyethers undergo complexation with the cations releasing the counter ion in the medium. Therefore, if the salt is an acid salt, say KHSO4, K+ ion will be first trapped by the crown ether followed by the release of HSO4 −. Again, in the case of an aqueous medium, HSO4 − will produce hydronium ion (H3O+), thereby making the medium acidic (). We have very efficiently used this idea for the synthesis of various substituted imidazoles.
Scheme 1. Potassium ion being trapped by the six oxygen atoms in the 18-crown-6 core and generation of hydronium ion in an aqueous medium.
In continuation of our search for the synthesis of biologically important heterocycles (Citation41–44), here we describe the synthesis of 2,4,5-tri- and 1,2,4,5-tetrasubstituted imidazoles in an aqueous medium under moderate reaction condition.
Results and discussion
Initially, we started the condensation of benzil (1 mmol), benzaldehyde (1 mmol), and ammonium acetate (3 mmol) in water (5 mL) at room temperature for 24 h in the absence of catalyst but the desired product was not formed at all. Then the same reaction mixture was refluxed on an oil bath for an extended period of time (24 h) which led to very poor yield (15%) of 2,4,5-trisubstituted imidazole (, entry 2). Then, it was thought worthwhile to carry out the reaction in the presence of comparatively available crown ethers (18C6 and DB18C6) and acidic salts (KHSO4 and NaHSO4). After a thorough screening with the above-mentioned crown ethers and salts, it was revealed that heating the reaction mixture at 60°C for 4 h yields the best result (92% of isolated yield, , entry 6) in the presence of 15 mol% of both DB18C6 and KHSO4. The details of the results obtained are given in .
Table 1. Condensation of benzil, benzaldehyde, and ammonium acetate in the presence of crown ethers and salts in an aqueous medium.
Once the reaction was standardized, our first attempt was the synthesis of various 2,4,5-trisubstituted imidazoles with DB18C6 and KHSO4 in water (), and the results are depicted in .
Scheme 2. Synthesis of 2,4,5-trisubstituted imidazoles in an aqueous medium using DB18C6 and KHSO4 (15 mol%).
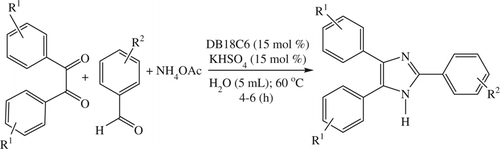
Table 2. Synthesis of 2,4,5-trisubstituted imidazoles in an aqueous medium using DB18C6 and KHSO4 (15 mol%).
On examination of we find that almost all of the 2,4,5-trisubstituted imidazoles were produced in high yields. A wide range of aromatic aldehydes bearing either electron donating or electron withdrawing substituents and different substituted benzils underwent this one-pot, three-component cyclocondensation to produce the products. Aliphatic aldehydes gave the corresponding imidazole in lower yield (52%) than did aromatic aldehydes while diacetyls gave lower yield of the product (65%) than that of other benzils. This is because of lower conjugation in the products than in the aromatic counterparts.
The same methodology was applied for the synthesis of 1,2,4,5-tetrasubstituted imidazoles via the one-pot, four-component condensation of an aldehyde, a benzil, a primary aromatic amine (as well as benzyl amine), and an ammonium acetate (), and the products were obtained in excellent yields in almost all cases except in the case of diacetyl where the yield is little lower (64%). The utility of this reaction was justified using various structurally different aldehydes and primary aromatic amines ().
Scheme 3. Synthesis of 1,2,4,5-tetrasubstituted imidazoles in an aqueous medium using DB18C6 and KHSO4 (15 mol%).
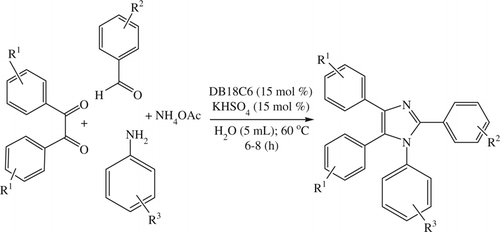
Table 3. Synthesis of 1,2,4,5-tetrasubstituted imidazoles in an aqueous medium using DB18C6 and KHSO4 (15 mol%).
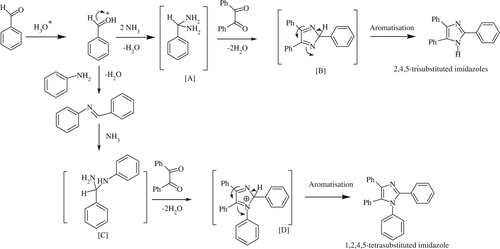
The mechanism of the formation of trisubstituted imidazoles involves protonation of the aldehydic carbonyl, diamine intermediate [A] formation with two molecules of ammonia from ammonium acetate and condensation with one molecule of diketone compound to form intermediate [B] that subsequently aromatized to produce the final products. In the case of tetrasubstituted imidazoles, the mechanism proceeds through the formation of the imine of the aromatic aldehydes with aromatic amines (as it is more stable than the imine of aldehydes and ammonia). This imine subsequently forms the diamine intermediate with ammonia and the rest of the mechanism remains the same. Because of the formation of the more stable imine intermediate in the case of four-component reaction of benzil, ammonium acetate, aromatic aldehydes, and aromatic primary amines, 1,2,4,5-tetrasubstituted imidazoles are exclusively formed (in place of the mixture of tri- and tetrasubstituted imidazoles). The detail of the mechanism is given in .
Scheme 4. Proposed mechanism for the DB18C6-KHSO4 catalyzed synthesis of substituted imidazoles formation.
After extraction by ethylacetate, the aqueous part containing DB18C6 and KHSO4 can be recycled at least six times for imidazole synthesis without substantial loss in the yield of the reaction. Therefore, the catalytic efficiency is quite high.
Experimental
General procedure for the trisubstituted imidazole formation
To a mixture of aldehyde (1 mmol), benzil (1 mmol), and ammonium acetate (3 mmol) in water (5 mL) were added DB18C6 (15 mol%) and KHSO4 (15 mol%), and the mixture was stirred at room temperature for 15 min. The resulting mixture was heated on a water bath at 60°C for the stipulated time mentioned in . When the reaction was complete as monitored by TLC, the reaction mixture was extracted with EtOAc (3×10 mL) and dried over anhydrous Na2SO4. After removal of the solvent in vacuo, the pure products were obtained by direct crystallization from ethanol.
After extraction by ethylacetate, the aqueous part containing DB18C6 and KHSO4 can be reused at least six times for the imidazole synthesis. This recyclability of the catalyst increases its catalytic efficiency.
For large-scale preparation, aldehyde (10 mmol), benzil (10 mmol), ammonium acetate (30 mmol), water (20 mL), DB18C6 (15 mol%), and KHSO4 (15 mol%) were taken in a round-bottom flask (100 mL) and stirred at room temperature for 30 min. The resulting mixture was heated on a water bath at 60°C until the reaction was completed (monitored by TLC). After the completion of the reaction, the reaction mixture was extracted with EtOAc (3×20 mL) and dried over anhydrous Na2SO4. After removal of the solvent in vacuo, the pure products were obtained by direct crystallization from ethanol.
General procedure for the tetrasubstituted imidazole formation
In the case of tetrasubstituted imidazoles, aldehyde (1 mmol), benzil (1 mmol), ammonium acetate (1.5 mmol), aromatic primary amines (1 mmol), DB18C6 (15 mol%), and KHSO4 (15 mol%) were taken in water (5 mL), and the rest of the procedure remains the same. The final products were obtained by extraction with ethyl acetate, solvent removal in vacuo and crystallization from ethanol.
For large-scale preparation of tetrasubstituted imidazoles, aldehyde (10 mmol), benzil (10 mmol), primary amines (10 mmol), ammonium acetate (15 mmol), water (20 mL), DB18C6 (15 mol%), and KHSO4 (15 mol%) were taken in a round- bottom flask (100 mL) and stirred at room temperature for 30 min. The resulting mixture was heated on a water bath at 60°C until the reaction was complete (monitored by TLC). After the completion of the reaction, the reaction mixture was extracted with EtOAc (3×20 mL) and dried over anhydrous Na2SO4. After removal of the solvent in vacuo, the pure products were obtained by direct crystallization from ethanol.
All the products gave satisfactory IR, 1H NMR, 13C NMR, and analytical data as given below.
4,5-Bis-(4-chlorophenyl)-2-(3′-nitrophenyl)-1H-imidazole (, entry 7)
The titled compound was obtained as a yellow solid (420 mg, 93%); R f=0.44 (25% ethylacetate/75% petroleum ether). M.P.: 314–315°C (MeOH); IR (KBr): 3072, 2964, 2376, 1527, 1348, 1091, and 834 cm−1. 1H NMR (300 MHz, d 6-DMSO): 12.84 (s, 1H), 8.65 (s, 1H), 8.21 (d, J=7.2 Hz, 1H), 7.91 (d, J=7.8 Hz, 1H), 7.46 (t, J=7.8 Hz, 1H), 7.40–7.10 (m, 8H). 13C NMR (75 MHz, d 6-DMSO): 148.3, 143.9, 136.9, 133.5, 132.8, 131.6, 131.2, 130.3, 130.1, 129.3, 128.9, 128.4, 122.7, 119.5, 117.8. Anal. calcd. for C21H13N3O2Cl2 (%): C 61.48, H 3.19, N 10.24. Found (%): C 61.36, H 3.27, N 10.28.
1-(4a-Chlorophenyl)-2-(4′-hydroxy-3′-methoxyphenyl)-4,5-diphenyl-imidazole (, entry 1)
The titled compound was obtained as a white solid (420 mg, 93%); R f=0.54 (25% ethylacetate/75% petroleum ether). M.P.: 196–198°C (EtOAc); IR (KBr): 3411, 3058, 1600, 1490, 1438, 1380, 1272, 1228, 1090, 1027, and 698 cm−1. 1H NMR (300 MHz, CDCl3): 7.60 (dd, J=8.0, 1.5 Hz, 2H, C4- and C5-phenyl protons), 7.33–7.18 (m, 8H, C4-, C5-phenyl protons), 7.13 (dd, J=8.6, 1.5 Hz, 3H, C2a-, C6a-, C2'–H), 6.99 (d, J=8.4 Hz, 2H, C3a-, C5a–H), 6.77–6.70 (m, 2H, C5'-, C6'–H), 3.74 (s, 3H, C3'–OCH3). 13C NMR (75 MHz, CDCl3): [146.9, 146.5, 146.4 (C4'-, C3'-, and C2)], 137.6 (C4), 135.6 (C1a), 134.2 (C4a), 133.6 (C5), 131.1 (2C, C4- and C5-phenyl carbons), [130.2, 130.1 (ipso to C4- and C5-, respectively)], 129.6 (2C, C2a, C6a), 129.3 (2C, C3a, C5a), 128.5 (2C, C4-, and C5-phenyl carbons), 128.2 (C4- or C5-phenyl carbon), 128.1 (2C, C4- or C5-phenyl carbons), 127.5 (2C, C4- or C5-phenyl carbons), 126.8 (C4- or C5-phenyl carbons), 122.5 (C6'), 121.4 (C1'), 114.3 (C2'), 112.0 (C5'), 55.8 (–OCH3). Anal. calcd. for C28H21N2O2Cl (%): C 74.25, H 4.67, N 6.19. Found (%): C 74.13, H 4.79, N 6.24.
1-(4a-Chlorophenyl)-2-(2′,5′-dimethoxyphenyl)-4,5-diphenyl-imidazole (, entry 2)
The titled compound was obtained as a white solid (429 mg, 92%); R f=0.75 (25% ethylacetate/75% petroleum ether). M.P.: 246–248°C (EtOAc); IR (KBr): 3341, 2936, 2372, 1602, 1522, 1484, 1441, 1221, 1178, 1045, and 694 cm−1. 1H NMR (300 MHz, CDCl3): 7.61 (dd, J=7.5, 1.2 Hz, 2H, C4-, C5-phenyl protons), 7.33–7.20 (m, 8H, C4-, C5-phenyl protons), 7.18–7.11 (m, 3H, C2a, C6a, and C6'-H), 6.95–6.86 (m, 3H, C3a, C5a, and C4'-H), 6.65 (d, J=9.0, 1H, C3'-H), 3.82 (s, 3H, C2'-OMe), 3.32 (s, 3H, C5'-OMe). 13C NMR (75 MHz, CDCl3): 153.4 (C2'), 150.9 (C5'), 145.0 (C2), 137.8 (C4), 135.6 (C1a), 133.7 (C5), 133.2 (C4a), 130.9 (2C, C4-, C5-phenyl carbons), 130.2 (ipso to C4), 129.4 (ipso to C5), 128.5 (2C, C2a, C6a), 128.4 (2C, C3a, C5a), 128.3 (2C, C4, C5-phenyl carbons), 128.1 (C4- or C5-phenyl carbon), 128.0 (2C, C4-, C5-phenyl carbons), 127.5 (2C, C4-, C5-phenyl carbons), 126.7 (C4- or C5-phenyl carbon), 119.8 (C1'), 117.1 (C6'), 116.9 (C4'), 111.8 (C3'), 55.8 (C2-OMe), and 55.0 (C5-OMe). Anal. calcd. for C29H23N2O2Cl (%): C 74.59, H 4.96, N 5.99. Found (%): C 74.45, H 5.12, N 6.08.
1-(4a-Chlorophenyl)-2-(3′,4′-dimethoxyphenyl)-4,5-diphenyl-imidazole (, entry 3)
The titled compound was obtained as a white crystalline solid (401 mg, 86%); R f=0.57 (25% ethylacetate/75% petroleum ether). M.P.: 172°C (EtOAc); IR (KBr): 3432, 3053, 2933, 2375, 1597, 1491, 1442, 1405, 1329, 1176, 1131, 1087, 1022, 845, 772, and 702 cm−1. 1H NMR (300 MHz, CDCl3): 7.62 (d, J=7.5 Hz, 2H, C4- and C5-phenyl protons), 7.26–7.17 (m, 8H, C4-, C5-phenyl protons), 7.14 (brd, J=5.7 Hz, 2H, C2a-H and C6a-H), 7.06 (s, 1H, C2'-H), 7.00 (d,J=8.1 Hz, 2H, C3a-H and C5a-H), 6.91 (d, J=8.4 Hz, 1H, C6'-H), 6.74 (d, J=8.4 Hz, 1H, C5'-H), 3.85 (s, 3H, C4'-OCH3), 3.74 (s, 3H, C3'-OCH3).13C NMR (75 MHz, CDCl3): 149.3 (C4'), 148.4 (C3'), 146.6 (C2), 137.8 (C4), 135.6 (C1a), 134.0 (C4a), 133.8 (C5), 130.9 (2C, C4-,C5-phenyl carbons), 130.2 (ipso to C4), 130.09 (ipso to C5), 129.6 (2C, C2a, C6a), 129.2 (2C, C3a, C5a), 128.4 (2C, C4-, C5-phenyl carbons), 128.1 (C4- or C5-phenyl carbon), 128.0 (2C, C4-, C5-phenyl carbons), 127.2 (2C, C4-, C5-phenyl carbons), 126.6 (C4- or C5-phenyl carbon), 122.4 (C1'), 121.7 (C6'), 112.1 (C2'), 110.6 (C5'), (55.5 and 55.7) (2×OCH3). Anal. calcd. for C29H23N2O2Cl (%): C 74.59, H 4.96, N 6.00. Found (%): C 74.43, H 5.04, N 6.07.
1-(4a-Chlorophenyl)-2-(4'-hydroxyphenyl)-4,5-diphenyl-imidazole (, entry 4)
The titled compound was obtained as an off-white solid (397 mg, 94%); R f=0.37 (25% ethylacetate/75% petroleum ether). M.P.: 290°C (MeOH); IR (KBr): 3034, 2935, 2593, 2490, 1604, 1488, 1440, 1398, 1276, 1165, 1094, 836, and 698 cm−1.
1H NMR (300 MHz, CDCl3): 9.72 (brs, 1H, –OH), 7.46 (d, J=7.2 Hz, 2H, C4,C5-phenyl protons), 7.33 (d, J=8.7 Hz, 2H, C2'-H and C6'-H), 7.27–7.25 (m, 3H, C3a-H, C5a-H, and one from either C4- or C5-phenyl protons), 7.21–7.11 (m, 9H, C3'-H, C5'-H, and seven from C4-, C5-phenyl protons), 6.67 (d, J=8.7H, 2H, C2a-H and C6a-H). 13C NMR (75 MHz, CDCl3): 157.8 (C4'), 146.7 (C2), 136.7 (C4), 135.9 (C5), 134.5 (C1a), 133.1 (C4a), 131.2 (2C, C4-, C5-phenyl carbons), 130.6 (2C, C2a, C6a), 130.54 (ipso to C4), 130.50 (ipso to C5), 130.0 (2C, C3a, C5a), 129.3 (2C, C4-, C5-phenyl carbons), 128.6 (2C, C4-, C5-phenyl carbons), 128.5 (2C, C4-, C5-phenyl carbons), 128.2 (2C, C4-, C5-phenyl carbons), 126.4 (2C, C2', C6'), 121.1 (C1'), 115.2 (C3', C5'). Anal. calcd. for C27H19N2OCl (%): C 76.68, H 4.53, N 6.62. Found (%): C 76.79, H 4.66, N 6.51.
2-(4'-Chlorophenyl)-1-(4a-nitrophenyl)-4,5-diphenyl-imidazole (, entry 5)
The titled compound was obtained as a yellow solid (428 mg, 95%); R f=0.96 (25% ethylacetate/75% petroleum ether). M.P.: 218°C (EtOAc); IR (KBr): 3432, 3066, 2365, 1597, 1521, 1492, 1412, 1345, 1095, 852, and 699 cm−1; 1H NMR (300 MHz, CDCl3): 8.10 (d, J=8.7 Hz, 2H, C3a-H and C5a-H), 7.56 (d, J=6.6 Hz, 2H, C4-, C5-phenyl protons), 7.33–7.09 (m, 14H, eight from C4-, C5-phenyl protons, C2a-, C6a-, C2'-, C3'-, C5'-, and C6'-H). 13C NMR (75 MHz, CDCl3): 147.1 (C4a), 145.6 (C2), 142.0 (C1a), 138.6 (C4), 135.3 (C5), 133.0 (C4'), 131.0 (2C, C4-, C5-phenyl carbons), 130.6 (ipso to C4), 130.3 (2C, C2', C6'), 129.4 (ipso to C5), 129.1 (2C, C2a, C6a), 128.8 (3C, C3', C5', and one carbon from either C4- or C5-phenyl carbon), 128.7 (2C, C4-, C5-phenyl carbons), 128.3 (2C, C4-, C5-phenyl carbons), 127.6 (C1'), 127.3 (2C, C4-, C5-phenyl carbons), 127.2 (C4- or C5-phenyl carbon), 124.5 (2C, C3a and C5a). Anal. calcd. for C27H18N3O2Cl (%): C 71.76, H 4.01, N 9.30. Found (%): C 71.85, H 4.23, N 9.21.
1-(4a-Nitrophenyl)-2-(4'-N,N-dimethylaminophenyl)-4,5-diphenyl-imidazole (, entry 6)
The titled compound was obtained as a brick red solid (396 mg, 86%); R f=0.73 (25% ethylacetate/75% petroleum ether). M.P.: 280–282°C (EtOAc); IR (KBr): 3436, 3071, 2853, 2797, 2369, 1606, 1521, 1488, 1346, 1198, and 697 cm−1; 1H NMR (300 MHz, CDCl3): 8.11 (d, J=8.7 Hz, 2H, C3a-H and C5a-H), 7.60 (dd, J=7.8 and 1.2 Hz, 2H, two protons from C4-, C5-phenyl protons), 7.33–7.21 (m, 8H, C4-, C5-phenyl protons), 7.18 (d, J=9.0 Hz, 2H, C2'-H and C6'-H), 7.11 (dd, J=8.1 and 1.8 Hz, 2H, C2a-H and C6a-H), 6.58 (d, J=9.0 Hz, 2H, C3'-H and C5'-H), 2.96 [s, 6H, –N(CH3)2]. 13C NMR (75 MHz, CDCl3): 150.6 (C4'), 147.7 (C4a), 146.8 (C2), 142.9 (C1a), 138.1 (C4), 133.5 (C5), 131.1 (2C, C4-, C5-phenyl carbons), 130.2 (2C, C2' and C6'), (123.0 and 129.4) (ipso to C4 and C5), 129.3 (2C, C2a and C6a), 128.7 (2C, C4-, C5-phenyl carbons), 128.5 (C4- or C5-phenyl carbon), 128.2 (2C, C4-, C5-phenyl carbons), 127.5 (2C, C4-, C5-phenyl carbons), 127.0 (C4- or C5-phenyl carbon), 124.4 (2C, C3a and C5a), 116.4 (C1'), 111.6 (2C, C3' and C5'), 40.1 [2C, –N(CH3)2]. Anal. calcd. for C29H24N4O2 (%): C 75.64, H 5.25, N 12.17. Found (%): C 75.51, H 5.33, N 12.29.
2-(2′, 5′-Dimethoxyphenyl)-1-(4a-nitrophenyl)-4,5-diphenyl-imidazole (, entry 7)
The titled compound was obtained as a yellow solid (396 mg, 83%); R f=0.64 (25% ethylacetate/75% petroleum ether). M.P.: 208–210°C (EtOAc); IR (KBr): 3426, 2940, 2375, 1598, 1522, 1492, 1347, 1223, 1040, and 698 cm−1; 1H NMR (300 MHz, CDCl3): 8.02 (d, J=9.0 Hz, 2H, C3a-H and C5a-H), 7.61 (d, J=6.9 Hz, 2H, C4-, C5-phenyl protons), 7.36–7.20 (m, 6H, C4-, C5-phenyl protons), 7.16 (brd, J=5.7 Hz, 3H, C2', and two other protons from C4- and C5-phenyl protons), 7.08 (d, J = 9.0 Hz, 2H, C2a-H and C6a-H), 6.93 (dd, J=9.0 and 3.0 Hz, 1H, C4'-H), 6.64 (d, J=9.0 Hz, 1H, C3'-H), 3.83 (s, 3H, C2-OCH3), 3.26 (s, 3H, C5'-OCH3). 13C NMR (75 MHz, CDCl3): 153.7 (C2'), 150.5 (C5'), 146.3 (C4a), 145.0 (C2), 142.7 (C1a), 138.3 (C4), 133.3 (C5), 130.9 (2C, C4-, C5-phenyl carbons), 129.7 (ipso to C4), 129.2 (ipso to C5), 128.8 (2C, C2a, C6a), 128.5 (C4- or C5-phenyl carbon), 128.1 (2C, C4-, C5-phenyl carbons), 127.7 (2C, C4-, C5-phenyl carbons), 127.5 (2C, C4-, C5-phenyl carbons), 127.0 (C4- or C5-phenyl carbon), 123.5 (2C, C3a, C5a), 119.2 (C1'), 117.3 (C6'), 117.1 (C4'), 111.9 (C3'), 55.8 (C2'-OC¯H3), 54.9 (C5'-OC¯H3). Anal. calcd. for C29H23N3O4 (%): C 72.95, H 4.85, N 8.80. Found (%): C 72.83, H 4.97, N 8.95.
2-(3′,4′-Dimethoxyphenyl)-1-(4a-methoxyphenyl)-4,5-diphenylimidazole (, entry 8)
The titled compound was obtained as a white solid (430 mg, 93%); R f=0.29 (25% ethylacetate/75% petroleum ether). M.P.: 174–176°C (EtOAc); IR (KBr): 2945, 2834, 2374, 2040, 1601, 1515, 1471, 1442, 1247, 1026, and 699 cm−1. 1H NMR (300 MHz, CDCl3): 7.60 (d, J=7.2 Hz, 2H, C4- and C5-phenyl protons), 7.30–7.10 (m, 8H,C4-, C5-phenyl protons), 7.05 (brs, 1H, C2'-H), 6.97 (d, J=8.4 Hz, 3H, C2a-H, C6a-H, and C6'-H), 6.76–6.69 (m, 3H, C3a-H, C5a-H, and C5'-H), 3.80 (s, 3H, C4a-OCH3), 3.71 and 3.68 [2s, 6H, C4'-OCH3 and C3'-OCH3]. 13C NMR (75 MHz, CDCl3): 159.0 (C4a), 148.9 (C4'), 148.2 (C3'), 146.7 (C2), 137.6 (C4), 134.4 (C5), 131.0 (2C, C4-, C5-phenyl carbons), (130.7, 130.6, and 129.9) (3C, C1a, C4 and C5 ipso carbons), 129.4 (2C, C2a, C6a), 128.1 (2C, C4-, C5-phenyl carbons), 127.9 (2C, C4-, C5-phenyl carbons),127.7 (C4- or C5-phenyl carbon), 127.2 (2C, C4-, C5-phenyl carbons), 126.3 (C4- or C5-phenyl carbon), 123.1 (C1'), 121.5 (C6'), 114.0 (2C, C3a, C5a), 111.3 (C2'), 110.6 (C5'), 55.6 (C4a-OMe), 55.5 (C4'-OMe), and 55.2 (C3'-OMe). Anal. calcd. for C30H26N2O3 (%): C 77.90, H 5.67, N 6.06. Found (%): C 77.81, H 5.75, N 6.14.
2-(4′-N,N-Dimethylaminophenyl)-1-(4a-methoxyphenyl)-4,5-diphenyl-imidazole (, entry 9)
The titled compound was obtained as an off-white solid (387 mg, 87%); R f=0.67 (25% ethylacetate/75% petroleum ether). M.P.: 224°C (EtOAc); IR (KBr): 3436, 2899, 2375, 1610, 1510, 1441, 1365, 1248, 818, and 699 cm−1; 1H NMR (300 MHz, CDCl3): 7.61 (d, J=7.5 Hz, 2H, C4-, C5-phenyl protons), 7.33 (d, J=8.7 Hz, 2H, C2'-H and C6'-H), 7.26–7.11 (m, 8H, C4-, C5-phenyl protons), 6.99 (d, J=8.7 Hz, 2H, C2a-H and C6a-H), 6.76 (d, J=8.7 Hz, 2H, C3a-H and C5a-H), 6.58 (d, J=8.7 Hz, 2H, C3'-H and C5'-H), 3.76 (s, 3H, C4a-OCH3), 2.93 [s, 6H, –N(CH3)2]. 13C NMR (75 MHz, CDCl3): 158.9 (C4a), 150.1 (C4'), 147.7 (C2), 137.5 (C4), 134.7 (C5), 131.2 (2C, C4-, C5-phenyl carbons), 131.1 (C1a), 130.3, and 130.2 (C4- and C5-ipso carbons), 129.8 (2C, C2', C6'), 129.6 (2C, C2a, C6a), 128.2 (2C, C4- and C5- phenyl carbons), 128.0 (2C, from C4- and C5-phenyl carbons), 127.6 (C4- or C5-phenyl carbon), 127.4 (2C, from C4- and C5-phenyl carbons), 126.3 (C4- or C5-phenyl carbon), 118.3 (C1'), 114.1 (2C, C3a, C5a), 111.6 (2C, C3', C5'), 55.3 (C4a-OC¯H3), 40.2 [2C, –N(CH3)2]. Anal. calcd. for C30H27N3O (%): C 80.87, H 6.11, N 9.43. Found (%): C 80.74, H 6.22, N 9.52.
1-(4a-Methoxyphenyl)-2-(4′-nitrophenyl)-4,5-diphenyl-imidazole (, entry 10)
The titled compound was obtained as a yellow solid (398 mg, 89%); R f=0.85 (25% ethylacetate/75% petroleum ether). M.P.: 198°C (EtOAc); IR (KBr): 3436, 2932, 2378, 1597, 1511, 1336, 1247, 853, and 699 cm−1. 1H NMR (300 MHz, CDCl3): 8.11 (d, J=8.7 Hz, 2H, C3'-H and C5'-H), 7.65 (d, J=9.0 Hz, 2H, C2'-H and C6'-H), 7.59 (dd, J=8.1 and 1.5 Hz, 2H, C4-, C5-phenyl protons), 7.32–7.13 (m, 8H, 4,5-phenyl protons), 7.03 (d, J=8.7 Hz, 2H, C2a-H and C6a-H), 6.82 (d, J=8.7 Hz, 2H, C3a-H and C5a-H), 3.80 (s, 3H, C4a-OCH3). 13C NMR (75 MHz, CDCl3): 159.7 (C4a), 147.07 (C4'), 144.3 (C2), 138.9 (C4), 136.2 (C1'), 133.5 (C5), 132.7 (C1a), 131.0 (2C, C4-, C5-phenyl carbons), 129.8 (C4- or C5-ipso carbon), 129.3 (2C, C2', C6'), 129.1 (2C, C2a, C6a), 129.0 (C4- or C5-ipso carbon), 128.5 (2C, C4-, C5-phenyl carbons), 128.4 (C4- or C5-phenyl carbon), 128.3 (2C, C4-, C5-phenyl carbons), 127.3 (2C, C2a, C6a), 127.1 (C4- or C5-phenyl carbon), 123.4 (2C, C3', C5'), 114.6 (2C, C3a, C5a), 55.4 (C4a–OC¯H3). Anal. calcd. for C28H21N3O3 (%): C 75.16, H 4.73, N 9.39. Found (%): C 75.03, H 4.89, N 9.44.
2-(3,4′-Dimethoxyphenyl)-1-(4a-methylphenyl)-4,5-diphenyl-imidazole (, entry 11)
The titled compound was obtained as a white solid (410 mg, 92%); R f=0.54 (25% ethylacetate/75% petroleum ether). M.P.: 162–164°C (EtOAc); IR (KBr): 3431, 3032, 2944, 2370, 1599, 1516, 1488, 1445, 1257, 1226, 1131, 1019, 770, and 699 cm−1.1H NMR (300 MHz, CDCl3): 7.64 (d, J=7.2 Hz, 2H from C4-, C5-phenyl protons), 7.27–7.15 (m, 8H from C4-, C5-phenyl protons), 7.07–7.02 (m, 4H, C2a-H, C6a-H, C2'-H, and C6'-H), 6.95 (d, J=8.4 Hz, 2H, C3a-H and C5a-H), 6.72 (d, J=9.0 Hz, 1H, C5'-H), 3.81 (s, 3H, C4'-OCH3), 3.67 (s, 3H, C3'-OCH3), 2.29 (s, 3H, C4a-CH3). 13C NMR (75 MHz, CDCl3): 148.9 (C4'), 148.1 (C3'), 146.5 (C2), 137.9 (C4a), 137.5 (C4), 134.5 (C1a), 134.2 (C5), 130.9 (2C, C4-, C5-phenyl carbons), 130.5, and 130.4 (2C, ipso to C4 and C5), 129.5 (2C, C2a, C6a), 128.1 (2C, C3a, C5a), 128.0 (2C, C4-, C5-phenyl carbons), 127.9 (2C, C4-, C5-phenyl carbons), 127.7 (C4- or C5-phenyl carbon), 127.1 (2C, C4-, C5-phenyl carbons), 126.3 (C4- or C5-phenyl carbon), 123.0 (C1'), 121.5 (C2'), 111.9 (C6'), 110.5 (C5'), 55.5 (C4'–OC¯H3), 55.3 (C3'–OC¯H3), 20.8 (C4a–C¯H3). Anal. calcd. for C30H26N2O2 (%): C 80.69, H 5.87, N 6.27. Found (%): C 80.54, H 5.99, N 6.35.
2-(4′-N,N-Dimethylaminophenyl)-1-(4a-methylphenyl)-4,5-diphenyl-imidazole (, entry 12)
The titled compound was obtained as a white solid (399 mg, 93%); R f=0.95 (25% ethylacetate/75% petroleum ether). M.P.: 234–236°C (EtOAc); IR (KBr): 2902, 1894, 1608, 1484, 1365, 1198, 816, 776, and 698 cm−1. 1H NMR (300 MHz, CDCl3): 7.62 (d, J=7.0 Hz, 2H, C4-, C5-phenyl protons), 7.34 (d, J=8.9 Hz, 2H, C2'-H and C6'-H), 7.30–7.13 (m, 8H, C4-, C5-phenyl protons), 7.07 (d, J=8.1 Hz, 2H, C2a-H and C6a-H), 6.96 (d, J=8.2 Hz, 2H, C3a-H and C5a-H), 6.59 (d, J=8.9 Hz, 2H, C3'-H and C5'-H), 2.95 [s, 6H, –N(CH3)2], 2.34 (s, 3H, C4a-CH3). 13C NMR (75 MHz, CDCl3): 150.1 (C4'), 147.6 (C2), 137.8 (C4a), 137.5 (C4), 134.8 (C1a), 134.6 (C5), 131.2 (2C, C4-, C5-phenyl carbons), 131.0 (ipso to C4), 130.1 (ipso to C5), 129.8 (2C, C2' and C6'), 129.6 (2C, C2a and C6a), 128.2 (2C, C3a and C5a), 128.2 (2C, C4- and C5-phenyl carbons), 128.0 (2C, C4-, C5-phenyl carbons), 127.6 (C4- or C5-phenyl carbon), 127.4 (C4- or C5-phenyl carbon), 126.3 (C4- or C5-phenyl carbon), 118.2 (C1'), 111.5 (2C, C3' and C5'), 40.1 [2C, –N(CH3)2], 21.1 (C4a-C¯H3). Anal. calcd. for C30H27N3 (%): C 83.88, H 6.34, N 9.78. Found (%): C 83.75, H 6.51, N 9.89.
1-(4a-Aminophenyl)-2-(4'-chlorophenyl)-4,5-diphenyl-imidazole (, entry 13)
The titled compound was obtained as a grey solid (379 mg, 90%); R f=0.64 (25% ethylacetate/75% petroleum ether). M.P.: 236–238°C (EtOAc); IR (KBr): 3382, 3051, 2275, 1623, 1515, 1293, 1092, 834, and 699 cm−1. 1H NMR (300 MHz, CDCl3): 7.41 (dd, J=8.1 and 1.8 Hz, 2H, C4-, C5-phenyl protons), 7.27 (dd, J=6.9 and 1.8 Hz, 2H, C2'-H and C6'-H), 7.14–6.90 (m, 10H, C3'-H, C5'-H, and eight protons from C4- and C5-phenyl protons), 6.62 (dd, J=6.8 and 2.1 Hz, 2H, C2a-H and C6a-H), 6.34 (dd, J=6.8 and 2.4 Hz, 2H, C3a-H and C5a-H), 3.84 (s, 2H, –NH2). 13C NMR (75 MHz, CDCl3): (146.7 and 145.5) (2C, C4a, C2), 137.3 (2C, C4, C5), (134.5 and 133.4 (2C, C1a, C4'), 131.5 (2C, ipso to C4, C5), 131.1 (2C, C4-, C5-phenyl carbons), 130.1 (2C, C2', C6'), 129.0, and 128.3 (6C, C2a, C6a, C3', C5', and two from C4- and C5-phenyl carbons), 128.2 (2C, C4-, C5-phenyl carbons), 128.1 (C4- or C5-phenyl carbon), 127.4 (2C, C4-, C5-phenyl carbons), 126.9 (C4- or C5-phenyl carbons), 126.8 (C1'), 115.1 (2C, C3a, C5a). Anal. calcd. for C27H20N3Cl (%): C 76.86, H 4.78, N 9.96. Found (%): C 76.75, H 4.91, N 9.82.
Conclusion
We have for the first time utilized potassium hydrogen sulfate in the presence of dibenzo-18-crown-6 (DB18C6) as an efficient and highly effective catalyst for the facile synthesis of a wide variety of tri- and tetrasubstituted imidazoles in an aqueous medium at moderate temperature. Mild reaction conditions, easy work-up procedure, excellent overall yield, and use of water as the green reaction medium are the major advantages of this methodology.
Acknowledgements
One of the authors (P.K.T.) thanks the University Grants Commission, New Delhi, for his fellowship (SRF). We thank the CAS Instrumentation Facility, Department of Chemistry, University of Calcutta, for spectral data. We also acknowledge the grant received from UGC-funded Major project, F. No. 37-398/2009 (SR) dated 11 January 2010.
References
- Parenty , A.D.C. ; Smith , L.V. ; Pickering , A.L. ; Long , D.L. ; Cronin , L. J. Org. Chem . 2004 , 69 , 5934 – 5946 .
- Tietze , L.F. Chem. Rev . 1996 , 96 , 115 – 136 .
- Bai , L. ; Wang , J.X. ; Zhang , Y. Green Chem . 2003 , 5 , 615 – 617 .
- Pironti , V. ; Colonna , S. Green Chem . 2005 , 7 , 43 – 45 .
- Nüchter , M. ; Ondruschka , B. ; Bonrath , W. ; Gum , A. Green Chem . 2004 , 6 , 128 – 141 .
- Herrerias , C.I. ; Xiaoquan , Y. ; Li , Z. ; Li , C.-J. Chem. Rev . 2007 , 107 , 2546 – 2562 .
- Lambardino , J.G. ; Wiseman , E.H. J. Med. Chem . 1974 , 17 , 1182 – 1188 .
- Philips , A.P. ; White , H.L. ; Rosen , S. Eur. Pat. Appl. EP 58890, 1982 .
- Lee , J.C. ; Laydon , J.T. ; McDonnell , P.C. ; Gallagher , T.F. ; Kumar , S. ; Green , D. ; McNulty , D. ; Blumenthal , M.J. ; Keys , J.R. ; Vatter , S.W.L. ; Strickler , J.E. ; McLaughlin , M.M. ; Siemens , I.R. ; Fisher , S.M. ; Livi , G.P. ; White , J.R. ; Adams , J.L. ; Young , P.R. Nature 1994 , 372 , 739 – 746 .
- Takle , A.K. ; Brown , M.J.B. ; Davies , S. ; Dean , D.K. ; Francis , G. ; Gaiba , A. ; Hird , A.W. ; King , F.D. ; Lovell , P.J. ; Naylor , A. ; Reith , A.D. ; Steadman , J.G. ; Wilson , D.M. Bioorg. Med. Chem. Lett . 2006 , 16 , 378 – 381 .
- de Laszlo , S.E. ; Hacker , C. ; Li , B. ; Kim , D. ; MacCoss , M. ; Mantalo , N. ; Pivnichny , J.V. ; Colwell , L. ; Koch , G.E. ; Cascieri , M.A. ; Hagmenn , W.K. Bioorg. Med. Chem. Lett . 1999 , 9 , 641 – 644 .
- Schmierer , R. ; Mildenberger , H. ; Buerstell , H. Preparation of phenylimidazoles as plant growth regulators, German Patent 361464, 1987 ; Chem. Abstr . 1988 , 108 , 37838 .
- Heeres , J. ; Backx , L.J.J. ; Mostmans , J.H. ; Van Custem , J. J. Med. Chem . 1979 , 22 , 1003 – 1005 .
- Antolini , M. ; Bozzoli , A. ; Ghiron , C. ; Kennedy , G. ; Rossi , T. ; Ursini , A. Bioorg. Med. Chem. Lett . 1999 , 9 , 1023 – 1026 .
- Wang , L. ; Woods , K.W. ; Li , Q. ; Barr , K.J. ; McCroskey , R.W. ; Hannick , S.M. ; Gherke , L. ; Credo , R.B. ; Hui , Y.-H. ; Marsh , K. ; Warner , R. ; Lee , J.Y. ; Zielinsky-Mozng , N. ; Frost , D. ; Rosenberg , S.H. ; Sham , H.L. J. Med. Chem . 2002 , 45 , 1697 – 1711 .
- Maier , T. ; Schmierer , R. ; Bauer , K. ; Bieringer , H. ; Buerstell , H. ; Sachse , B. 1-Substituted imidazole-5-carboxylic acid derivatives, their preparation and their use as biocides, US Patent 4,820,335, 1989 ; Chem. Abstr . 1989 , 111 19494w
- Dupont , J. ; de Souza , R.F. ; Suarez , P.A.Z. Chem. Rev . 2002 , 102 , 3667 – 3692 .
- Nara , S.J. ; Naik , P.U. ; Harjani , J.R. ; Salunkhe , M.M. Indian J. Chem . 2001 , 45B , 2257 – 2269 .
- Chowdhury , S. ; Mohan , R.S. ; Scott , J.L. Tetrahedron 2007 , 63 , 2363 – 2389 .
- Bourissou , D. ; Guerret , O. ; Gabbai , F.P. ; Bertrand , G. Chem. Rev . 2000 , 100 , 39 – 92 .
- Wolkenberg , S.E. ; Wisnoski , D.D. ; Leister , W.H. ; Wang , Y. ; Zhao , Z. ; Lindsley , C.W. Org. Lett . 2004 , 6 , 1453 – 1456 .
- Shaabani , A. ; Rahmati , A. ; Farhangi E. ; Badri , Z. Catal. Commun . 2007 , 8 , 1149 – 1152 .
- Shaabani , A. ; Rahmati , A. J. Mol. Catal. A: Chem . 2006 , 249 , 246 – 248 .
- Wang , L.-M. ; Wang , Y.-H. ; Tian , H. ; Yao , Y.-F. ; Shao , J.-H. ; Liu , B. J. Fluorine Chem . 2006 , 127 , 1570 – 1573 .
- Heravi , M.M. ; Bakhtiari , K. ; Oskooie , H.A. ; Taheri , S. J. Mol. Catal. A: Chem . 2007 , 263 , 279 – 281 .
- Kokare , N.D. ; Sangshetti , J.N. ; Shinde , D.B. Synthesis 2007 , 39 , 2829 – 2834 .
- Kidwai , M. ; Mothsra , P. ; Bansal , V. ; Somvanshi , R.K. ; Ethayathulla , A.S. ; Dey , S. ; Singh , T.P. J. Mol. Catal. A: Chem . 2007 , 265 , 177 – 182 .
- Mohammed , A.E. ; Kokare , N.D. ; Sangshetti , J.N. ; Shinde , D.B. J. Korean Chem. Soc . 2007 , 51 , 418 – 422 .
- Chary , M.V. ; Keerthysri , N.C. ; Vupallapati , S.V.N. ; Lingaiah , N. ; Kantevari , S. Catal. Commun . 2008 , 9 , 2013 – 2017 .
- Sun , Y.-F. ; Huang , W. ; Lu , C.-G. ; Cui , Y.-P. Dyes Pigm . 2009 , 81 , 10 – 17 .
- Khosropour , A.R. Ultrason. Sonochem . 2008 , 15 , 659 – 664 .
- Sharma , S.D. ; Hazarika , P. ; Konwar , D. Tetrahedron Lett . 2008 , 49 , 2216 – 2220 .
- Kidwai , M. ; Ruby , S.S. ; Rastogi , S. Bull. Korean Chem. Soc . 2005 , 26 , 2051 – 2053 .
- Kantevari , S. ; Vuppalapati , S.V.N. ; Biradar , D.O. ; Nagarapu , L. J. Mol. Catal. A: Chem . 2007 , 266 , 109 – 113 .
- Shelke , K.F. ; Sapkal , S.B. ; Shingare , M.S. Chin. Chem. Lett . 2009 , 20 , 283 – 287 .
- Balalaie , S. ; Arabanian , A. ; Hashtroudi , M.S. Monatsh. Chem . 2000 , 131 , 945 – 948 .
- Siddiqui , S.A. ; Narkhede , U.C. ; Palimkar , S.S. ; Daniel , T. ; Lahoti , R.J. ; Srinivasan , K.V. Tetrahedron 2005 , 61 , 3539 – 3546 .
- Xia , M. ; Lu , Y.-D. J. Mol. Catal. A: Chem . 2007 , 265 , 205 – 208 .
- Chauveau , E. ; Marestin , C. ; Schiets , F. ; Mercier , R. Green Chem . 2010 , 12 , 1018 – 1022 .
- Ochiai , M. Coord. Chem. Rev . 2006 , 250 , 2771 – 2781 .
- Mukhopadhyay , C. ; Tapaswi , P.K. Tetrahedron Lett . 2008 , 49 , 6237 – 6240 .
- Mukhopadhyay , C. ; Tapaswi , P.K. ; Butcher , R.J. Tetrahedron Lett . 2010 , 51 , 1797 – 1802 .
- Mukhopadhyay , C. ; Tapaswi , P.K. ; Drew , M.G.B. Tetrahedron Lett . 2010 , 51 , 3944 – 3950 .
- Mukhopadhyay , C. ; Tapaswi , P.K. ; Butcher , R.J. Org. Biomol. Chem . 2010 , 8 , 4720 – 4729 .
- Wang , L.M. ; Wang , Y.H. ; Tian , H. ; Yao , Y.F. ; Shao , J.H. ; Liu , B. J. Fluorine Chem . 2006 , 127 , 1570 – 1573 .
- Frantz , D.E. ; Morency , L. ; Soheilli , A. ; Murry , J.A. ; Grabowski , E.J.J. ; Tillyer , R.D. Org. Lett . 2004 , 6 , 843 – 846 .
- Samai , S. ; Nandi , G.C. ; Singh , P. ; Singh , M.S. Tetrahedron 2009 , 65 , 10155 – 10161 .