Abstract
A series of E-(1)-(6-benzoyl-3,5-dimethylfuro[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones were synthesized by the Claisen–Schimdt reaction of 2-acetyl-3,5-dimethyl-6-benzoyl benzodifuran with aromatic/heteroaromatic aldehydes under solvent-free microwave irradiation. All these compounds were characterized by means of their IR, 1H NMR, and 13C NMR spectra and elemental analysis. All the compounds were screened for their antibacterial activity.
Introduction
Benzofuran derivatives have been reported to possess a wide variety of biological activities. They have been reported to possess antimicrobial (1–3), antitumor Citation4, and anti-inflammatory Citation5 activity. Chalcones are α,β-unsaturated ketones and have great existence in plant kingdom. They have been reported to possess antioxidant Citation6, antimalarial Citation7, anti-inflammatory Citation8, anticancer Citation9, antiviral Citation10, and antimicrobial activity Citation11. Benzodifurans exhibit interesting biological activities such as antibacterial Citation12, antifungal Citation12, anti-implantation Citation12, and antiviral activity Citation13. In view of the biological activities exhibited by benzofurans, benzodifurans, and α,β-unasaturated ketones, we have synthesized some new E-(1)-(6-benzoyl-3,5-dimethylfuro[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones (3a–g).
Results and discussion
Recently microwave-induced organic reaction enhancement Citation14 chemistry has gained popularity as a nonconventional technique for rapid organic synthesis; it is eco-friendly and economical and is believed to be a step toward green chemistry. The solvent-less reactions offer a number of advantages: solvents are often expensive, toxic, and difficult to remove in the case of aprotic solvents with high boiling points. The most popular way of synthesis of chalcones is the Claisen–Schmidt condensation of an appropriate acetophenone with benzaldehyde in the presence of aqueous bases such as NaOH Citation15, KOH Citation16, Ba(OH)2 Citation17, magnesium t-butoxide Citation18, potassium carbonate Citation19, alumina Citation20, and piperidine Citation21. We have developed a simple and solvent-free method for the synthesis of E-(1)-(6-benzoyl-3,5-dimethylfuro[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones (3a–g). The mild reaction conditions, clean reaction profiles, minimal side products, and cost efficiency render this approach as a useful alternative to the existing methods.
The required starting material 2-acetyl-3,5-dimethyl-6-benzoyl benzodifuran was synthesized by reacting 5-acetyl-2-benzoyl-6-hydroxy-3-methyl benzofuran Citation22 Citation23 with 2-chloroacetone in the presence of anhydrous K2CO3 under conventional and microwave irradiation methods.
The targeted molecules E-Citation1-(6-benzoyl-3,5-dimethylfuro[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones (3a–g) were synthesized in excellent yields by condensing 2-acetyl-3,5-dimethyl-6-benzoyl benzodifuran with aromatic/heteroaromatic aldehydes in the presence of sodium hydroxide in ethanol under conventional heating and microwave irradiation. The reaction time has been brought down from hours to minutes using microwave-assisted synthesis, with improved yields. The antibacterial activity of synthesized E-(1)-(6-benzoyl-3,5-dimethylfuro[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones (3a–g) was carried out by using cup plate method ( ).
Experimental
Melting points were determined in open capillaries and are uncorrected. The purity of the compounds was checked routinely by the silica gel F254 (Merck). Microwave reactions were carried out in the Milestone MultiSYNTH microwave system. IR spectra were recorded on Shimadzu FTIR-8400s spectrophotometer. 1H NMR and 13C NMR spectra were recorded on Avance 300 spectrometer, and mass spectra were recorded on Shimadzu mass spectrometer. Elemental analysis was determined by using ThermoFinnigan CHNS analyzer.
Experimental procedure
General procedure for the synthesis of 2-acetyl-3,5-dimethyl-6-benzoyl benzodifuran (2)
Microwave irradiation method
A thoroughly blended mixture of 5-acetyl-2-benzoyl-6-hydroxy-3-methyl benzofuran (1) (5 mmol), chloroacetone (5 mmol), and anhydrous potassium carbonate (3g) was taken in a quartz tube and inserted into a Teflon vial with screw capped and then subjected to microwave irradiation at the constant temperature 120°C for 4 min. Progress of the reaction was monitored by thin-layer chromatography (TLC). After completion of the reaction it was diluted with cold water, and the precipitate formed was filtered, washed with water, and recrystallized from dichlormethane (DCM)/pet ether (40–60°C) in 1:3 ratio.
Conventional heating method
A solution of 5-acetyl-2-benzoyl-6-hydroxy-3-methyl benzofuran (1) (0.03 mol), chloroacetone (0.03 mol), K2CO3 (30 g), and acetone (150 mL) was refluxed for 8 h. Progress of the reaction was monitored by TLC. After completion of the reaction, acetone was distilled under vacuum and chilled water was added to the residue. The precipitate formed was filtered, washed with water, and recrystallized from DCM/pet ether (40–60°C) in 1:3 ratio.
General procedure for the synthesis of E-(1)-(6-benzoyl-3,5-dimethylfuro[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones (3a–g)
Microwave irradiation method
A thoroughly blended mixture of 2-acetyl-3,5-dimethyl-6-benzoyl benzodifuran (2) (0.001 mol), appropriate aromatic/heteroaromatic aldehydes (0.001 mol), and powdered NaOH (0.001 mol) was taken in a quartz tube and inserted into a Teflon vial with screw capped and then subjected to microwave irradiation at the constant temperature 90°C for 4–5 min. Progress of the reaction was monitored by TLC. After completion of the reaction it was diluted with cold water and acidified with dil. HCl. The precipitate formed was filtered, washed with water, and recrystallized from methanol as a yellow solid.
Conventional heating method
A mixture of 2-acetyl-3,5-dimethyl-6-benzoyl benzodifuran (2) (0.001 mol), appropriate aromatic/heteroaromatic aldehydes (0.001 mol), NaOH (0.001 mol), and EtOH (10 mL) was taken in a 50 mL round-bottom flask and then it was refluxed for 6–8 h. Progress of the reaction was monitored by TLC. After completion of the reaction it was diluted with cold water and acidified with dil. HCl. The precipitate formed was filtered, washed with water, and recrystallized from methanol as a yellow solid.
Table 1. Physical and analytical data for E-(1)-(6-benzoyl-3,5-dimethylfuro[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones.
IR, NMR, and mass spectral data of 2 and (3a-g)
2: IR (KBr): 3076, 2923, 1672 (>C = O), 1643 (>C = O), 1622, 1600, 1568, 1502, 1494, 1448, 1423, 1361, 1315, 1263, 1218, 1182, 1166, 1141, 1128, 1110, 1076, 1010, 958, 941, 923 cm−1. 1H NMR (300 MHz, DMSO-d 6): 2.63 (s, 3H, CH3), 2.67 (s, 3H, CH3), 2.69 (s, 3H, CH3), 7.50–7.65 (m, 4H, C8–H, C3, C4, C5, –H), 7.86 (s, 1H, C4–H), 8.05 (d, 2H, ortho protons of benzoyl ring). MS: [M]+ m/z=332 (100%).
3a: IR (KBr): 3058, 2962, 2923, 2852, 1654 (>C = O), 1623, 1604, 1566, 1496, 1448, 1425, 1371, 1357, 1325, 1259, 1201, 1163, 1105, 1016, 937 cm−1. 1H NMR (300 MHz, DMSO-d 6): 2.66 (s, 3H, CH3), 2.70 (s, 3H, CH3), 7.20 (s, 1H, Ar–H), 7.43 (d, 1H, α-H), 7.79 (s, 1H, Ar–H), 7.60–7.91 (m, 8H, Ar–H), 7.87 (d, 1H, β-H). MS: [M + H]+ m/z=421 (100%). Analysis calculated for C28H20O4 (%): C 80.0, H 4.76. Found (%): C 81.4, H 4.85.
3b: IR (KBr): 3141, 3089, 3033, 2941, 1643 (>C = O), 1618, 1581, 1560, 1496, 1467, 1440, 1380, 1353, 1319, 1290, 1180, 1151, 1058, 1022, 983 cm−1. 1H NMR (300 MHz, DMSO-d 6): 2.70 (s, 3H, CH3), 2.79 (s, 3H, CH3), 7.22 (s, 1H, Ar–H), 7.35 (d, 1H, =CHα),7.59–7.90 (m, 7H, Ar–H), 7.93 (d, 2H, Ar–H), 8.10 (d, 2H, Ar–H), 8.13 (d, 1H, β-H). MS: [M + H]+ m/z=455 (90%). Analysis calculated for C28H19O4Cl (%): C 73.92, H 4.18. Found (%): C 74.04, H 4.25.
3c: IR (KBr): 3058, 3004, 2929, 2853, 1654 (>C = O), 1643, 1623, 1596, 1566, 1512, 1446, 1423, 1373, 1357, 1325, 1255, 1197, 1172, 1105, 1027, 1012, 979 cm−1. 1H NMR (300 MHz, DMSO-d 6): 2.71 (s, 3H, CH3), 2.77 (s, 3H, CH3), 3.87 (s, 3H, OCH3), 6.95 (d, 1H, =CHα), 7.25 (s, 1H, Ar–H), 7.52–7.78 (m, 7H, Ar–H), 7.83 (s, 1H, Ar–H), 7.9 (d, 1H, β-H), 8.11 (d, 2H, Ar–H). 13C NMR (300 MHz, DMSO-d 6): ▵ 9.35, 9.55, 9.85, 45.93, 54.96, 55.41, 94.93, 95.25, 113.81, 114.30, 114.58, 119.50, 122.96, 123.82, 124.47, 126.49, 126.93, 128.55, 129.22, 130.29, 132.83, 137.39, 142.16, 143.59, 148.18, 149.05, 153.93, 154.22, 157.72, 161.64, 180.50, 184.93. MS: [M + H]+ m/z=451 (100%). Analysis calculated for C29H22O5 (%): C 77.33, H 4.88. Found (%): C 77.48, H 4.85.
3d: IR (KBr): 3093, 3058, 2958, 2916, 1654 (>C = O), 1637, 1623, 1604, 1560, 1477, 1467, 1425, 1373, 1325, 1259, 1211, 1184, 1107, 1008, 970, 927 cm−1. 1H NMR (300 MHz, DMSO-d 6): 2.60 (s, 3H, CH3), 2.68 (s, 3H, CH3), 6.70 (d, 1H, β-H, furyl), 7.43 (d, 1H, β′-H, furyl), 7.11 (d, 1H, =CHα), 7.21 (s, 1H, Ar–H), 7.56–7.71 (m, 4H, 3, Ar–H and 1H, =CHβ), 7.93–7.96 (d, 2H–Ar–H), 8.05 (s, 1H, Ar–H), 8.29 (s, 1H, Ar–H). MS: [M + H]+ m/z=410 (20%). Analysis calculated for C26H18O5 (%): C 76.09, H 4.39. Found (%): C 76.18, H 4.39.
3e: IR (KBr): 3056, 2954, 2920, 1649 (>C = O), 1623, 1604, 1596, 1566, 1510, 1492, 1446, 1425, 1396, 1373, 1323, 1259, 1211, 1184, 1163, 1103, 1010, 937 cm−1. 1H NMR (300 MHz, DMSO-d 6): 2.65 (s, 3H, CH3), 2.78 (s, 3H, CH3), 7.61 (s, 1H, Ar–H), 7.63 (d, 1H, =CHα), 7.68–7.83 (m, 3H, Ar–H), 7.88 (d, 2H, Ar–H), 7.94 (d, 1H, =CHβ), 8.02–8.33 (m, 5H, Ar–H), 8.66 (d, 2H, Ar–H). MS: [M + H]+ m/z=471 (100%). Analysis calculated for C32H22O4 (%): C 81.70, H 4.68. Found (%): C 81.63, H 4.76.
3f: IR (KBr): 3056, 2960, 2916, 1647 (>C = O), 1623, 1596, 1560, 1541, 1506, 1446, 1411, 1373, 1323, 1257, 1010, 937 cm−1. 1H NMR (300 MHz, DMSO-d 6): 2.61 (s, 3H, CH3), 2.69 (s, 3H, CH3), 7.97 (s, 1H, Ar–H), 7.40 (d, 1H, =CHα), 7.52–7.73 (m, 14H, 13Ar–H and 1H, =CHβ), 8.0 (d, 2H, Ar–H), 8.33 (s, 1H, Ar–H), 9.45 (s, 1H, triazolyl-H). MS: [M + H]+ m/z=563 (100%). Analysis calculated for C37H26O4N2 (%): C 79.0, H 4.62, N 4.98. Found (%): C 78.89, H 4.62, N 5.05.
3g: IR (KBr): 3056, 2950, 2916, 1649 (>C = O), 1623, 1596, 1566, 1533, 1500, 1446, 1413, 1323, 1257, 1105, 1008, 958, 937 cm−1. 1H NMR (300 MHz, DMSO-d 6): 2.64 (s, 3H, CH3), 2.72 (s, 3H, CH3), 7.71 (s, 1H, Ar–H), 7.42 (d, 1H, =CHα), 7.55–7.68 (m, 12H, 11Ar–H and 1H, =CHβ), 8.01 (d, 2H, Ar–H), 8.39 (s, 1H, Ar–H), 9.5 (s, 1H, triazolyl-H). MS: [M + H]+ m/z=642 (40%). Analysis calculated for C37H25O4N2Br (%): C 69.24, H 3.90, N 4.36. Found (%): C 69.10, H 3.95, N 4.41.
Antibacterial activity
All the compounds were screened for their antibacterial activity Citation24 against bacterial strains such as Bacillus subtilis (ATCC-6633), Staphylococcus aureus (ATCC-29737), Escherichia coli (ATCC-10536), and Pseudomonas aeruginosa (ATCC-27853) using streptomycin, tetracycline, chloramphenicol, and carbenicillin as standard drugs. The activity was determined using the cup-plate agar diffusion method by measuring the inhibition zone in millimeters. Nutrient agar was used as a culture medium. A 1 mg/mL solution in dimethylformamide was used. The agar medium was inoculated with bacterial cultures tested. After 24 h of incubation at 37°C, the diameter of inhibition zone (in millimeters) was measured. The results of the antibacterial activity are given in . Among the compounds screened, 3a, 3b, 3f, and 3g showed good activity against all bacteria. The remaining compounds (3c, 3d, and 3e) were found to be moderately active against all bacteria.
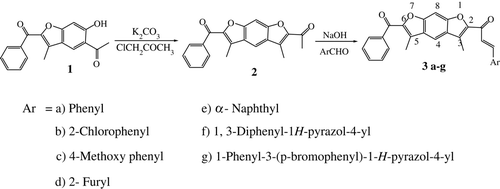
Table 2. Antibacterial activity of compounds 3a–g and inhibition zones.
Conclusion
In conclusion, we have successfully synthesized E-(1)-(6-benzoyl-3,5-dimethyl furo[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones (3a–g) under solvent-free microwave irradiation conditions. In this method there is no need to recover, purify, and reutilize the solvent, which reduces the pollution arising from such operations. This methodology provides an easily facile, economical, and environmentally benign synthesis in which the reaction time is reduced with better yields. The E-(1)-(6-benzoyl-3,5-dimethylfuro[3′,2′:4,5]benzo[b]furan-2-yl)-3-(aryl)-2-propen-1-ones exhibit moderate antibacterial activity.
Acknowledgements
The authors are thankful to the Head of the Department of Chemistry, Osmania University Hyderabad, India, and the Chairman and Managing Director, Sven Genetech Limited, Hyderabad, India, for providing laboratory facilities to carry out the research work. They also thank the Director, IICT, Hyderabad, India, for providing necessary instrumental facilities.
References
- Aruna Kumar , D.B. ; Prakash , G.K. ; Kumaraswamy , M.N. ; Nandeshawarappa , B.P. ; Sheringara , B.S. ; Mahadevan , K.M. Ind. J. Chem . 2007 , 46B , 336 – 343 .
- Manna , K. ; Agarwal , Y.K. Bioorg. Med. Chem. Lett . 2009 , 19 10 , 2688 – 2692 .
- Urzúa , A. ; Rezende , M.C. ; Mascayano , C. ; Vásquez , L. Molecules , 2008 , 13 10 , 882 – 891
- Hayakawa , I. ; Shioya , R. ; Agatsuma , T. ; Furukawa , H. ; Naruto , S. ; Sugano , Y. Bioorg. Med. Chem. Lett . 2004 , 14 , 455 – 458 .
- Santana , L. ; Teijeira , M. ; Uriate , E. ; Teran , C. ; Linares , B. ; Viller , R. ; Laguna , R. ; Cano , E. Eur. J. Pharm. Sci . 1999 , 7 , 161 – 166
- Anto , J.R. ; Sukumaraan , K. ; Kuttan , G. ; Rao , M.N.A. ; Subbaraju , V. ; Kuttan , R. Cancer Lett . 1995 , 97 , 33 – 37
- Soon , S.L. ; Hye-Sook , K. ; Dong-Ung , L. Bull. Korean Chem. Soc . 2007 , 28 12 , 2495 – 2497
- Avila , H.P. ; Smania , E.F.A. ; Monache , F.D. ; Smania , A. Jr. Bioorg. Med. Chem . 2008 , 16 , 9790 – 9794 .
- Shen , J. ; Chang , T.L. ; Loti , T.S. ; Jing , R.W. ; Horng , K. , Chun-Nan , L. Eur. J. Med. Chem . 2005 , 40 , 103 – 112
- Yu , D.C. ; Panfilova , L.V. ; Boreko , E.I. Pharm. Chem . 1982 , 16 , 103 – 105 .
- Batovska , D. ; Parushev , S. ; Stamboliysa , B. ; Tsvetkova , I. ; Ninova , M. ; Najdenski , H. Eur. J. Med. Chem . 2009 , 44 , 2211 – 2218 .
- Krishna Murthy , K.S. ; Rajitha , B. ; Kanakalingeswara R.M. Ind. J. Chem . 2003 , 42B , 425—428
- Viano , I. ; Dianzani , C. ; Gia , O. ; Gastaldi , S. ; Genazzani , E. Drugs, Exp. Clin. Res . 1985 , 11 12 , 865 – 867 .
- Ahluwalia , V.K. ; Kidwai , M. New Trends in Green Chemistry ; Anamaya : New Delhi , , India , 2004 ; p 59 (and references cited therein) .
- Satyanarayana , M. ; Tiwari , P. ; Tripathi , B.K. ; Srivastava , A.K. ; Pratap , R. Bioorg. Med. Chem . 2004 , 12 , 883 – 889 .
- Bu , X. ; Zhao , L. ; Li , Y. Synthesis 1997 , 1246 – 1248
- Sinisterra , J.V. ; Garcia-Raso , A. ; Cabello , J.A. ; Marians , J.M. Synthesis , 1984 , 502—503
- Rochus , W. ; Kickuth , R. German Patent 1 095 832, 1957
- Guthrie , J.L. ; Rabjhon , N. J. Org. Chem . 1957 , 22 , 176 – 179 .
- Varma , R.S. ; Kabalka , G.W. ; Evans , L.T. ; Pagani , R.M. Synth. Commun . 1985 , 15 , 279 – 284 .
- Trivedi , J.C. ; Bariwal , J.B. ; Upadhyay , K.D. ; Naliapara , Y.T. ; Joshi , S.K. ; Pannecouque , C.C. ; Clercq , E.D. ; Shah , A.K. Tetrahedron Lett . 2007 , 48 , 8472 – 8474 .
- Sharada , J. ; Ratna Kumari , Y. ; Kanakalingeswara , R.M. Ind. J. Chem . 1986 , 25B , 334 – 337 .
- Vishnu Vardhan Reddy , K. ; Sampath Rao , P. ; Ashok , D. Synth. Commun 2000 , 30 10 , 1825 – 1836 .
- The United States Pharmacopeia Biological tests and assay – An official monograph of USP 25th ed. , Rockwille, MD , 2001