Abstract
An environmentally benign, room temperature aqueous synthesis of pyrazoles by the condensation of hydrazines/hydrazides with 1,3-diketones using Amberlyst-70 as a recyclable catalyst is described. The use of resinous, non-toxic, thermally stable and inexpensive Amberlyst-70 as a heterogeneous catalyst and simple reaction workup are the additional eco-friendly attributes of this synthetic protocol.
Introduction
It has been proved that corrosive mineral acids are not environment friendly. They are not safe to handle and easy to dispose. This is the reason why safer and eco-friendly alternative solid acid catalysts like acidic zeolites, clays, sulfated zirconia and ion exchange resins are being developed for their applications in catalysis Citation1. There are various advantages for using these solid catalysts instead of a liquid catalyst. These advantages include reduced equipment corrosion, easy experimental and product isolation procedures, less contamination in waste streams and recyclability Citation2 Citation3. Recent decades have witnessed an exponential growth in the application of heterogeneous catalysis to carry out chemical transformations as a consequence of its significance in terms of environmental, economical and practical aspects Citation4 Citation5. Polystyrene sulfonic acid (PSSA) resin catalysts like Amberlyst-15, Amberlyst-70, Nafion, etc., are adding to the ever-growing portfolio of highly active solid acid catalysts. They have been used commercially as solid acid catalysts for hydration and etherification of olefins, dehydration of alcohol, alkylation of phenol, hydrolysis of ester and other acid catalyzed reactions Citation1 Citation6–9.
Amongst the many five-membered heterocycles, considerable interest has been focused on the pyrazole nucleus, which is known to possess a broad spectrum of biological properties such as hypoglycemic, cytotoxic, anti-malarial and antidepressant activities Citation10–14. Pyrazoles also possess important properties such as antitumor Citation15, cyclin-dependent kinase inhibitors Citation16, monoamine oxidase-B inhibitors and as atypical antipsychotic Citation17. In addition to these, pyrazole nucleus is a core structure of numerous biologically active compounds and highly popular drugs like Celebrex Citation18 and Viagra Citation19.
Synthetically pyrazole derivatives can be obtained by the action of hydrazine/hydrazides on 1,3-dicarbonyls Citation20 Citation21. Other synthetic routes which do not involve 1,3-dicarbonyls have also been developed Citation22–27. A few efficient routes have been reported. However, most of these utilize circuitous route, require longer reaction time and are often carried out in organic solvents Citation28 Citation29. Use of toxic organic solvents, expensive catalysts, harsh reaction conditions and disposal of catalyst/ reaction waste leaves scope for further development of new environmentally clean syntheses. Water as a solvent, is inexpensive, environmentally benign and often give better yields with completely new reactivity Citation30 Citation31. Non-toxic, non-corrosive and non-flammable nature and relatively high vapor pressure as compared to organic solvents are favorable individuality to render water as a sustainable alternative Citation32 Citation33.
Many researchers have explored the utility of various heterogeneous catalysts such as Nafion (NR-50), Scolecite, Montmorillonite K-10, etc., for different chemical transformations Citation34–37. Despite of having high thermal stability over other ion exchange resin catalysts, Amberlyst-70 is still under-explored for the synthesis of heterocycles. Furthermore, relatively few reports have appeared on the use of heterogeneous catalyst for the synthesis of pyrazoles Citation38–41. Recently, Polshettiwar and Varma evaluated the catalytic efficiency of PSSA in the synthesis of pyrazoles Citation21. Moreover, the same group reported the use of nano-organocatalyst for the synthesis of pyrazoles Citation42. We have recently reported the utility of Amberlyst-70 for aqueous Biginelli reaction Citation43. In our quest for greener synthetic pathway, we herein report, the use of Amberlyst-70 as a recyclable catalyst for a simple, facile and environmentally benign aqueous synthesis of pyrazoles. This is the first report on the use of Amberlyst-70 for the synthesis of pyrazoles.
Results and discussion
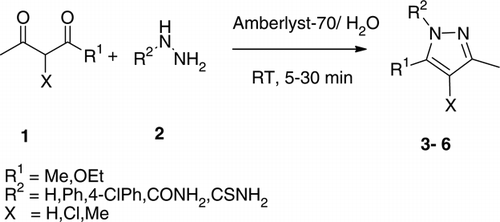
Pyrazoles were synthesized in aqueous medium by the action of hydrazines/hydrazides on 1,3-dicarbonyls using Amberlyst-70 as a solid heterogeneous catalyst at room temperature (Scheme 1).
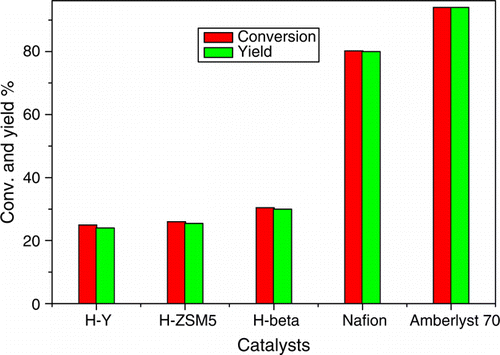
During initial exploratory reaction, condensation of acetylacetone and phenyl hydrazine was investigated to establish the feasibility of our strategy and to optimize reaction conditions using various solid acid catalyst like zeolites (H-Y, H-ZSM5, H-beta), Nafion resin (SAC-13) and Amberlyst-70. represents the effect of different catalysts on the condensation of acetylacetone and phenylhydrazine to pyrazole. The catalyst Amberlyst-70 gave an exclusively 94% selectivity for pyrazole at 94% conversion of acetylacetone within 15 minute of reaction time. Completion of reaction was monitored by TLC and the further quantitative analysis was done by gas chromatography (GC). The yield and the rate of reaction were superior in the case of Amberlyst-70 as compared to that of other catalysts.
Figure 2. The effect of different catalyst on the condensation of acetylacetone and phenylhydrazine.
The zeolites gave lower yield for pyrazole in comparison with Amberlyst-70. For zeolites, we have got the selectivity for pyrazole in the range of 25–30%. In case of Nafion resin (SAC-13), yield was around 80%. This yield was lower than that obtained with Amberlyst-70.
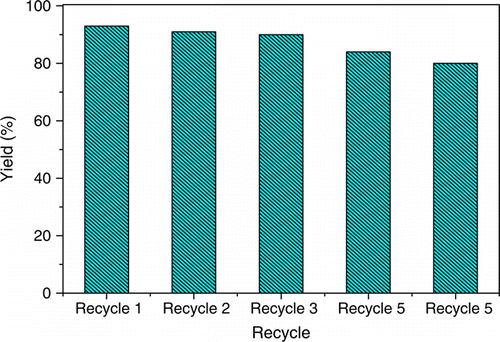
We also studied the recyclability of Amberlyst-70 for the reaction of acetylacetone with phenyl hydrazine for five cycles (). During filtration of the reaction mixture, the catalyst was easily separated, washed with ether, dried at 60 °C for 4 hr and used for recycle study. Each time, the used catalyst gave 2–3% lower yield than the previous one.
With the above optimized reaction condition, pyrazole synthesis using Amberlyst-70 in aqueous medium was probed for the synthesis of a wide variety of substituted pyrazoles ().
Table 1. Amberlyst-70 catalyzed synthesis of pyrazoles at room temperature in aqueous medium.
All aforementioned reactions () proceeded expeditiously at room temperature and delivered good yields with range of 1,3-dicarbonyl compounds. In all the cases the pure product was isolated by simple filtration or extraction without use of any chromatography or cumbersome reaction workup. The recrystallization of solid products afforded pure compounds. The described protocol eliminates the use of organic solvent except for the extraction and recrystallization. It is noteworthy to mention that these reactions are working well in aqueous medium without using any phase transfer catalyst.
General procedure for the synthesis of pyrazoles
1,3-Dicarbonyl compound (1 mmol), hydrazine/hydrazide (1.1 mmol) and catalytic amount of Amberlyst-70 (0.050 g) were suspended in water (15 mL). The reaction mixture was stirred at 30 °C for 5–30 minute. The completion of the reaction was monitored by TLC. The reaction mixture was analysed for its contents by GC (Agilent, 6090N) equipped with FID and capillary column (HP-5). Confirmation of the product has been done from GC-mass spectroscopy analysis and comparing with the retention times of the authentic samples. After confirmation, the reaction mixture was filtered, washed with excess of water. The product obtained was extracted with ethanol (the catalyst being separated at the same time) to afford almost pure pyrazoles in excellent yield.
Conclusion
Thus, we have demonstrated an elegant protocol for the synthesis of substituted pyrazoles using Amberlyst-70, which proceeds efficiently at room temperature in aqueous medium without use of any organic solvent. The adopted procedure is simple, rapid and eco-friendly due to the easy experimental and product isolation procedures; hence it is useful addition to the existing methods. The use of resinous, non-toxic, thermally stable and inexpensive Amberlyst-70 as a heterogeneous catalyst, simple reaction workup are the additional eco-friendly attributes of this synthetic protocol.
References
- Harmer , M.A. ; Sun , Q. App. Catal. A 2001 , 221 , 45 and references cited therein .
- Clark , J.H. , Catalysis of Organic Reactions by Supported Reagents ; VCH: NY, USA, 1994; Clark, J.H. Ed. Chemistry of Waste Minimization; 1995 ; pp 86 – 115 .
- Sheldon , R.A. ; van Bekkum , H. , Fine Chemicals Through Heterogeneous Catalysis ; Wiley-VCH : Weinheim , , Germany , 2002 .
- Corma , A. Chem. Rev . 1997 , 97 , 2373 2420 .
- Clark , J.H. ; Macquarrie , D.J. Chem. Soc. Rev . 1996 , 303 310 .
- Sharma , M.M. React. Funct. Polym . 1995 , 26 , 3 23 .
- Chakrabarti , A. ; Sharma , M.M. React. Funct. Polym . 1993 , 20 , 1 45 .
- Tejero , J. ; Cunill , F. ; Iborra , M. ; Izquierdo , J.F. ; Fite , C. J. Mol. Catal. A: Chem . 2002 , 182–183 , 541 – 559 .
- Siril , P.F. ; Davison , A.D. ; Randhawa , J.K. ; Brown , D.R. J. Mol. Catal. A: Chem . 2007 , 267 , 72 – 78
- Wright , J.B. ; Dulin , W.E. ; Markillie , J.H. J. Med. Pharm. Chem . 1964 , 7 , 102 .
- Smith , D. ; Forest , A. ; Dulin , W.E. J. Med. Pharm. Chem . 1965 , 8 , 350 – 353
- LeBlanc , R. ; Dickson , J. ; Brown , T. ; Stewart , M. ; Pati , H.N. ; VanDerveer , D. ; Arman , H. ; Harris , J. ; Pennington , W. ; Volt , H.L. , Jr. ; Lee , M. Bioorg. Med. Chem . 2005 , 13 , 6025 – 6034 .
- Kumar , S. ; Kumar , G. ; Kapoor , M. ; Surolia , A. Synth. Commun . 2006 , 36 , 215 – 226 .
- Palaska , E. ; Aytemir , M. ; Uzbay , T. ; Erol , D. Eur. J. Med. Chem . 2001 , 36 , 539 – 543 .
- Deasi , D. ; Sinha , I. ; Null , K. ; Wolter , W. ; Suckow , M.A. ; King , T. ; Amim , S. ; Sinha , R. Int. J. Cancer 2010 , 127 , 230 – 238 .
- Lin , R. ; Chiu , G. ; Yu , Y. ; Connollly , P.J. ; Li , S. ; Lu , Y. ; Adams , M. ; Fuentes-Pesquera , A.R. ; Emanual , S.L. ; Greenberger , L.M. Biorg. Med. Chem. Lett . 2007 , 17 , 4873 – 4877 .
- Gokhan-Kelekci , N. ; Yabanoglu , S. ; Kupeli , E. ; Salgin , U. ; Ozgen , O. ; Ucar , G. ; Yesilada , E. ; Kendi , E. ; Yesilada , A. ; Bilgin , A.A. Biorg. Med. Chem . 2007 , 15 , 5775 – 5786 .
- Penning , T.D. ; Talley , J.J. ; Bertenshaw , S.R. ; Isakson , P.C. J. Med. Chem . 1997 , 40 , 1347 – 1365 .
- Terrett , N.K. ; Bell , A.S. ; Brown , D. ; Ellis , P. Biorg. Med. Chem. Lett . 1996 , 6 , 1819 – 1824 .
- Katritzky , A.R. 1985 . Handbook of Heterocyclic Chemistry , 416 New York : Pergamon .
- Polshettiwar , V. ; Varma , R.S. Tetrahedron Lett . 2008 , 49 , 397 – 400 and references therein .
- Bhat , B.A. ; Puri , S.C. ; Qurishi , M.A. ; Dhar , K.L. ; Qazi , G.N. Synth. Commun . 2005 , 35 , 1135 – 1142 .
- Bishop , B.C. ; Brands , K.M.J. ; Gibb , A.D. ; Kennedy , D.J. Synthesis 2004 , 1 , 43 – 52 .
- Ahmed , M.S.M. ; Kobayashi , K. ; Mori , A. Org. Lett . 2005 , 7 , 4487 – 4489 .
- Deng , X. ; Mani , N.S. Org. Lett . 2006 , 8 , 3505 – 3508 ; Deng, X.; Mani, N.S. J. Org. Chem. 2008, 73, 2412–2415 .
- Martin , R. ; Rivero , M.R. ; Buchwald , S.L. Angew. Chem. Int. Ed . 2006 , 45 , 7079 – 7082 .
- Palakodety , R.K. ; Empati , R.S. ; Mongin , F. Tetrahedron Lett . 2008 , 49 , 6768 – 6772 .
- Liu , Z. ; Shi , F. ; Martinze , P.D.G. ; Raminelli , C. ; Larock , R.C. J. Org. Chem . 2008 , 73 , 219 – 226 .
- Hari , Y. ; Tsuchida , S. ; Sone , R. ; Aoyama , T. Synthesis 2007 , 3371 – 3375 .
- Li , C.J. ; Chen , L. Chem. Soc. Rev . 2006 , 35 , 68 – 82 .
- Molteni , V. ; Hamilton , M.M. ; Mao , L. ; Crane , C.M. ; Termin , A.P. ; Wilson D.M. Synthesis 2002 , 12 , 1669 – 1674 .
- Polshettiwar , V. ; Varma , R.S. Chem. Soc. Rev ., 2008 , 37 , 1546 – 1557 .
- V. , Polshettiwar , Aqueous Microwave Assisted Chemistry: Synthesis and Catalysis ; RSC : UK , 2010 .
- Ramalinga , K. ; Vijayalakshmi , P. ; Kaimal , T.N.B. Synth. Lett . 2001 , 863 – 865 .
- Shinde , S.V. ; Jadhav , W.V. ; Lande , M.K. ; Gadekar , L.S. ; Arbad , B.R. ; Kondre , J.M. ; Karade , N.N. Catal. Lett . 2008 , 125 , 57 – 61 .
- Joseph , J.K. ; Jain , S.L. ; Sain , B. J. Mole. Catal. A: Chem . 2006 , 247 , 99 – 102 .
- Yadav , J.S. ; Subba Reddy , B.V. ; Jagan Reddy , E. ; Ramalingam , T. J. Chem. Res . 2000 , 354 – 355 .
- Boullet , F.T. ; Klein , B. ; Hamelin , J. Synthesis , 1986 , 409 – 411 .
- Chen , X. ; She , J. ; Shang , Z.C. ; Wu , J. ; Zhang , P. Synth. Commun . 2009 , 39 , 947 – 957 .
- Landge , S.M. ; Schmidt , A ; Outerbridge , V. ; Török , B. Synth. Lett . 2007 , 10 , 1600 – 1604 .
- Braibante , M.E.F. ; Braibante , H.T.S. ; Tavares , L.C. ; Rohte , S.F. ; Costa , C.C. ; Morel , A.F. ; Stuker , C.Z. ; Burrow , R.A. Synthesis 2007 , 16 , 2485 – 2490 .
- Polshettiwar , V. ; Varma , R.S. Tetrahedron 2010 , 66 , 1091 – 1097 .
- Chandak , H.S. ; Lad , N.P. ; Upare , P.P. Catal. Lett . 2009 , 131 469 – 473 .