Abstract
Quinoxaline derivatives have been synthesized in high yields in the presence of p-dodecylbenzensulfulfonic acid as an inexpensive, nontoxic, and efficient Bronsted acid-surfactant-combined catalyst in water at room temperature.
Introduction
Performing organic reactions in water has attracted much attention over the past decades due to its numerous advantages such as being considerably safe, nontoxic, environmentally friendly, and cheap Citation1–5. In addition, reactions in water can facilitate access to different reactivity and selectivity patterns compared with those observed in common organic solvents due to its unique physical and chemical properties.
Quinoxalines and its derivatives are an important class of nitrogen-containing heterocyclic compounds and have shown a broad spectrum of biological activities such as antiviral Citation4, antibacterial Citation6, anti-inflammatory Citation7, and anticancer Citation8, which have made them privileged structures in pharmacologically active compounds.
Various methods have been reported for synthesis of quinoxaline derivatives via a condensation process catalyzed by zeolites Citation9, CuSO4·5H2O Citation10, cerium ammonium nitrate Citation11, Zn [(L)praline] in HOAc Citation12, molecular iodine in DMSO Citation9 or CH3CN Citation13, ionic liquids Citation14, montmorillonite K-10 Citation15, H6P2W18O62·2H2O Citation16, Yb(OTf)3 Citation17, or using Bi Citation18, Pd(OAc)2 Citation19, and MnO2 Citation20, via a oxidative coupling reaction. Some of these processes suffer from some limitations such as use of volatile organic solvents, low product yields, expensive reagents, tedious work-up procedures, or harsh reaction conditions.
Results and discussion
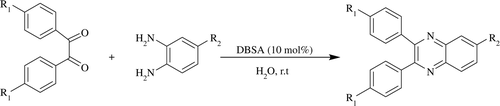
DBSA Citation21 as a Bronsted acid-surfactant-combined catalyst (BASC) has been known to play an important role not only for the activation of the substrate but also for the solubilization of organic substrates by the formation of colloidal dispersions. DBSA works well for dehydration reactions such as the esterification and etherification in water Citation22, synthesis of bis(indol-3-yl)alkanes Citation23, xanthenes Citation24 Citation25, 3-arylmethyl-4-hydroxycoumarins Citation26, selective α-aminoallylation Citation27, as well as in polymerization reactions Citation28–30. Herein, we wish to report a green and efficient method for the synthesis of quinoxaline derivatives in good to excellent yields by the condensation of 1,2-diamines with 1,2-dicarbonyl compounds catalyzed by DBSA.
Our studies were initiated with a model condensation reaction, between benzil (PhCOCOPh) and 1,2-diaminobenzene in H2O at room temperature, using a catalytic amount of DBSA ().
To optimize the reaction conditions, we carried out the model reaction in various solvents. As shown in , condensation reaction catalyzed by DBSA(10 mol%) in various solvents including water, ethanol, ethanol/water, methanol, n-hexane, acetone, acetonitril was investigated resulting in the product being 96%, 80%, 84%, 84%, 74%, 75%, 40% isolated in yields, respectively. Thus, water is obviously the best choice for this reaction.
Table 1. Effect of solvents on the synthesis of 2,3-diphenylquinoxaline.a
In addition to DBSA, we screened the effect of other surfactants such as cetyl trimethylammonium bromide (CTAB), tetradecyl trimethyl ammonium bromide (TTAB), dodecyl trimethyl ammonium bromide (DTAB), and Esterquat, on the time and yield of the model reaction. The results in show that reducing surface tension has a great effect on the rate of the reaction so that it progresses to great extent in the absence of any Bronsted acid (Entries 2–5).
Table 2. Effect of different surfactants on the reaction time and yield of 2,3-diphenylquinoxaline.a
The effect of the amounts of DBSA on the yields of condensation reaction in water has been shown in. It showed that when a mixture of 1 mmol benzil, and 1 mmol o-phenylenediamine was stirred for 24 h in the absence of DBSA, no product was detected (Entry 1), which showed that the catalyst should be necessary for this condensation reaction.
Table 3. Effect of amount of DBSA on the reaction times and yields.a
Afterward, we selected 5 mol% of DBSA to catalyze the model reaction and found that the desired quinoxaline was obtained in 92% yield. The reaction worked well when the amount of DBSA was increased to 10 mol%. (, Entry 3). When the amount of catalyst increased to 20%, the yields did not increase noticeably (, Entry 4). Thus, 10 mol% DBSA was chosen as the optimum amount of catalyst.
Besides DBSA, we also used catalytic amounts of other inorganic and organic acids such as HCl and p-toluenesulfonic acid (PTSA) for synthesis of quinoxaline from o-phenylenediamine and benzil in water as solvent. The results are depicted in . From these results, it is clear that DBSA is more efficient catalyst in water than common Bronsted acids.
Table 4. Effect of different Bronsted acids on the synthesis of 2,3-diphenylquinoxaline.a
After optimization, in model condensation reaction, the scope and the generality of the present method was demonstrated by the condensation of various substituted 1,2-diaminobenzen with 1,2-dicarbonyl compounds using catalytic amount of DBSA in water and the results are presented in .
Table 5. Synthesis of quinoxaline derivatives using DBSA as a BASC.
Experimental
General method for preparation of quinoxaline in water
A mixture of 1,2-diketone (1 mmol), 1,2-diamino derivatives (1 mmol), and p-dodecylbenzensulfonic acid (DBSA) (10 mol%) in water (3 mL) was stirred at room temperature. Progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the precipitated solid which is almost a pure product was filtered. Further purification could be done by recrystallization from a suitable solvent like ethanol or tetrahydrofuran (THF). All of the obtained quinoxalines are known compounds and identified by 1HNMR and melting points compared with the literature values.
Conclusions
In conclusion, we developed a simple, eco-friendly, convenient, and efficient procedure for synthesis of quinoxaline from the various 1,2-dicarbonyl and 1,2-diamin compounds using catalytic amount of DBSA under mild reaction conditions at room temperature.
Moreover, the procedure offers several advantages including high yields, operational simplicity, cleaner reactions and minimal environmental impact, which make it a useful process for the synthesis of quinoxaline derivatives.
Acknowledgements
We gratefully acknowledge the financial support received for this research work from the Research Council of Semnan University. Also we would like to acknowledge and thank the Condor company for their generous support.
References
- Breslow , R. Acc. Chem. Res. 1991 , 24 , 159 – 164 .
- Engberts , J.B.F.N. ; Blandamer , M.J. Chem. Commun . 2001 , 1701 – 1708 .
- Kobayashi , S. ; Manabe , K. Pure Appl. Chem. 2000 , 72 1373 – 1380 .
- Loriga , M. ; Moro , P. ; Sanna , P. ; Paglietti , G. ; Zanetti , S. Farmaco 1997 , 52 , 531 – 537 .
- Lindström , U.M. Chem. Rev . 2002 , 102 , 2751 – 2772 .
- Kumar , P. ; Kuamr , A. ; Mohan , L.J. ; Makrandi , J.K. Bull. Korean Chem. Soc . 2010 , 31 , 3304 – 3308 .
- Kumar , A. ; Verma , A. ; Chawla , G. ; Vaishali . Int. J. Chem. Tech. Res . 2009 , 1 , 1177 – 1181 .
- Gavara , L. ; Saugues , E. ; Alves , G. ; Debiton , E. ; Anizon , F. ; Moreau , P. Eur. J. Med. Chem . 2010 , 45 , 5520 – 5526 .
- Bhosale , R.S. ; Sarda , S.R. ; Ardhapure , S.S. ; Jadhav , W.N. ; Bhusare , S.R. ; Pawar , R.P. Tetrahedron Lett . 2005 , 46 , 7183 – 7186 .
- Heravi , M.M. ; Taheri , S. ; Bakhtiari , K. ; Oskooie , H.A. Catal. Commun . 2007 , 8 , 211 – 214 .
- More , S.V. ; Sastry , M.N.V. ; Yao , C.-F. Green Chem . 2006 , 8 , 91 – 95 .
- Heravi , M.M. ; Tehrani , M.H. ; Bakhtiari , K. ; Oskooie , H.A. Catal. Commun . 2007 , 8 , 1341 – 1344 .
- More , S.V. ; Sastry , M.N.V. ; Wang , C.-C. ; Yao , C.-F. Tetrahedron Lett . 2005 , 46 , 6345 – 6348 .
- Meshram , H.M. ; Ramesh , P. ; Santosh Kumar , G. ; Chennakesava Reddy , B. Tetrahedron Lett . 2010 , 51 , 4313 – 4316 .
- Huang , T.-K. ; Wang , R. ; Shi , L. ; Lu , X.-X. Catal. Commun . 2008 , 9 , 1143 – 1147 .
- Huang , T.K. ; Shi , L. ; Wang , R. ; Guo , X.Z. ; Lu , X.X. Chin. Chem. Lett . 2009 , 20 , 161 – 164 .
- Wang , L. ; Liu , J. ; Tian , H. ; Qian , C. Synth. Commun . 2004 , 34 1349 .
- Antoniotti , S. ; Duñach , E. Tetrahedron Lett . 2002 , 43 , 3971 – 3973 .
- Robinson , R.S. ; Taylor , R.J.K. Synlett 2005 , 1003 – 1005 .
- Raw , S.A. ; Wilfred , C.D. ; Taylor , R.J.K. Org. Biomol. Chem . 2004 , 2 , 788 – 796 .
- Shiri , M. ; Zolfigol , M.A. Tetrahedron 2009 , 65 , 587 – 598 .
- Aoyama , N. ; Kobayashi , S. Chem. Lett . 2006 , 35 , 238 .
- Peng , Y.Y. ; Zhang , Q.L. ; Yuan , J.J. ; Cheng , J.P. Chin. J. Chem . 2008 , 26 , 2228 – 2232 .
- Jin , T.S. ; Liu , L.B. ; Yin , Y. ; Zhao , Y. ; Li , T.S. Lett. Org. Chem . 2006 , 3 , 591 – 596 .
- Jin , T.S. ; Zhang , J.S. ; Guo , T.T. ; Wang , A.Q. ; Li , T.S. Synthesis 2004 , 2001 – 2005 .
- Palmisano , G. ; Tibiletti , F. Penoni , A. ; Colombo , F. ; Tollari , S. ; Garella , D. ; Tagliapietra , S. ; Cravotto , G. Ultrason. Sonochem . 2011 , 18 , 652 – 660 .
- Kobayashi , S. ; Hirano , K. ; Sugiura , M. Chem. Commun . 2005 , 104 – 106 .
- Caramyshev , A.V. ; Lobachov , V.M. ; Selivanov , D.V. ; Sheval , E.V. ; Vorobiev , A.K. ; Katasova , O.N. ; Polyakov , V.Y. ; Makarov , A.A. ; Sakharov , I.Y. Biomacromolecules 2007 , 8 , 2549 – 2555 .
- Ding , X. ; Han , D. ; Wang , Z. ; Xu , X. ; Niu , L. ; Zhang , Q. J. Colloid Interface Sci . 2008 , 320 , 341 – 345 .
- Massoumi , B. ; Aghili , H. ; Entezami , A. J. Chin. Chem. Soc . 2009 , 56 , 741 – 747 .