Abstract
One-pot silica promoted selective aromatization of ring-A of cholesterol using N-bromosuccinimide (NBS) has been performed. The structure of the compound has been characterized by spectral analysis (UV, FT-IR, NMR, and Mass).
Keywords:
Introduction
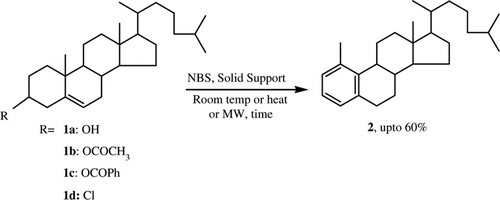
Selective aromatization of steroidal skeleton has been regarded as an efficient and useful chemical transformation both in terms of synthetic and biological consideration. Many of the steroidal hormones have been aromatized by using various transformation protocols. Although Marker and co-workers Citation1 first reported the transformation of steroidal nucleus into its aromatic analog, it was the work of Inhoffen and Zuhhdarff which firmly established the involvement of such type of chemical transformation Citation2–5. They reported an acid-catalyzed dienone-phenol rearrangement of 1, 4-cholestadien-3-one to furnish a phenol entity, the structure of which was later on confirmed by others Citation2–8. Again, Romo and his group showed a thermal transformation of 1, 4-cholestadien-3-one into aromatic derivatives and the dieneone-phenol rearrangement in the cholesterol series Citation9 Citation10. Hanson and Organ Citation11 Citation12 have reported a steroidal aromatization reaction which includes a transformation of cholesterol into A-ring aromatized product 4-methyl-19-norcholesta-1, 3, 5(10)-triene on treatment with 1, 3-dibromo-5, 5-dimethylhydantoin. But they were unable to isolate the compound in its pure form Citation11. They reported NBS as a poorer alternative to hydantoin. Urbanos and co-workers Citation13 Citation14 showed an efficient selective transformation of steroids into aromatized derivatives by an electrophilic ruthenium complex. However, in order to aromatize steroidal compounds selectively, people have used several steroidal enones Citation15, hydroxy ketones Citation16, epoxy steroids Citation10 Citation17 Citation18, dieneones Citation19–22, etc. as the starting material. Developmental studies in this area are still in progress Citation15 Citation21, Citation23–26. Microbial Citation27–32 as well as enzymatic Citation33 Citation34 steroidal aromatization has also been attempted, and the compounds are found to have potent biological implications. As a consequence of the importance given toward the selective aromatization of a steroidal skeleton, we started working in this area and finally could able to report herein a novel synthesis of A-ring aromatized cholesterol via a very simple one-pot solid phase reaction.
Results and discussion
A mixture of cholesterol (1a, 0.38 g, 1.0 mmol) and NBS (0.42 g, 5.0 mmol) in activated silica furnished a selective aromatic product 1-methyl-19-norcholesta-1,3,5(10)-triene, 2 (). To the best of our knowledge the isolated compound is being reported for the first time and it is actually isomeric to the compound reported by Hanson and Organ Citation11.
Compound 2 appeared as pale greenish yellow gum (46%, 0.17 g, 0.46 mmol) with characteristic odor which on standing (about 30 days at room temperature) slowly solidifies. The solidified mass was analyzed (TLC, IR, and NMR) to be a mixture of compounds which could not be isolated and moreover, it did not contain the aromatized product 2 (by comparison with the TLC, IR, and NMR spectra of the gummy sample Citation2 and its solidified mass).
The isolated product 2 (as a gum) was characterized on the basis of UV, IR, 1H, 13C NMR spectroscopy, mass spectrometry, and elemental analysis. The UV spectrum of 2 showed a peak, the pattern of which is similar to the benzenoid derivatives (λmax=277 nm, € = 2360). The IR spectrum showed absorptions at νmax 2951, 1462, 738, and 815 cm–1 characteristic for an aromatic ring. 1H NMR spectrum showed peaks at δ2.19 (3H, s, CH3), 6.97(1H, d, J =7.2 Hz, CH), 7.05(1H, t, J=7.5 Hz, CH), 7.17(1H, d, J=8.1 Hz, CH) ppm which correspond to the aromatic A-ring of the product. The 1H decoupled 13C NMR spectrum of 2 showed 27 distinct resonances of which six belong to the aromatic carbons. The mass spectrum displayed a molecular ion peak (M+) and base peak at m/z 366 (38%) and 365 (M+-H), respectively.
The reaction was attempted both on solid support as well as in solution phase. However, no aromatization was observed in solution phase.
For a preliminary study of optimization we performed the reaction using three different protocols, viz., (1) reaction at room temperature; (2) by the use of microwave irradiation (taking silica gel 60–120 mesh and F254, both); and (3) at elevated temperatures (taking only silica gel 60–120 mesh). Although the conversion is found to be rapid both in microwave irradiation and at higher temperature the yield was not satisfactory following either of the protocols. It was further established that the optimized time (in terms of percentage yield) at room temperature is 72 h, at thermal condition 5 min, and in microwave it was 5–7 min (150 W, 100°C) ( and ). The slow reaction rate of the transformation is quite obvious from the fairly longer optimized reaction time at room temperature. Investigation indicated that beyond 72 h there is a gradual decrease of percentage yield of the product (). This may be explained on the basis of the observation that beyond 72 h it slowly started solidifying and consequently analysis of the solid mass indicated that it is different from aromatized product 2 and finally identified as a mixture of compounds as stated above.
Table 1. Optimization of the reaction condition taking cholesterol and NBSa – at room temperature and MW irradiation.
Table 2. Optimization of the reaction conditiona taking cholesterol and NBSb – at elevated temperatures.
Optimization study for the amount of silica support (silica gel 60–120 mesh) for the above aromatization reaction was also performed, and it was established that 1 g mmol−1 of silica is best suitable for the transformation ().
Table 3. Optimization for the silica support a – taking cholesterol and NBSb.
Variation of solid supports () indicated that silica gel 60–120 mesh (used normally for column chromatography) was found to be the most effective support for the transformation. Silica gel F254 gave poor yield, alumina and KF-alumina did not yield the product (2) at all, and bentonite and 4 Å molecular sieves showed very little transformation. After each and every experiment the unreacted starting material, cholesterol, has been recovered quantitatively. Catalytic role of silica for the above transformation has also been tested (); other solid supports used in the study gave very poor yield of the product.
Table 4. Optimization for the solid supporta – taking cholesterol and NBSb.
Table 5. Recycling experiment using cholesterol and NBS catalyzed by silicaa.
Recyclability of the catalyst was tested by the following way – after the reaction is over, chloroform was added to the reaction mixture and the silica gel was filtered off followed by successive washings with methanol (×2), acetone (×1), and was activated (150°C/1 mm Hg, 1 h). The recovered activated silica was then used in a fresh transformation as the supporting medium for the reaction and it was found that upto six consecutive runs, there was no appreciable change in the percentage yield of the desired product. Thus silica may be considered as a very effective reusable solid support for this specific transformation ().
In order to generalize the findings, we extended the above reaction on another steroidal system, diosgenin, but surprisingly we could not isolate similar aromatic analog of diosgenin using NBS, although Sondheimer and his group Citation35 has reported its aromatization via another route.
Replacement of C-3 –OH by –Cl (1d) showed similar result but introduction of OAc (1b) gave better yield compared to cholesterol itself. On the other hand the benzoate derivative (1c) gave poor yield of the product ().When we carried out the same reaction on a 4-substituted cholesterol derivative, 4-β-hydroxycholesterol, we could not isolate the aromatic product. Thus the transformation cannot be correlated with nature as well as on the position of the substituent in ring-A of cholesterol.
Table 6. Effect of the 3-substituent on yield of 2.
Experimental
UV spectrum was measured on JASCO V-530 UV/VIS Spectrophotometer. Infrared spectrum was measured in neat with a Shimadzu FT-IR 8300 Spectrometer. The NMR spectra were recorded on a 300 MHz Bruker Avance FT-NMR spectrometer with CDCl3 as solvent and TMS as internal reference, chemical shifts are expressed as δ ppm. Mass spectrum was measured on a JEOL-AccuTOF JMS-T100LC Mass Spectrometer. Analytical-TLC was performed with silica gel plates using silica gel G for thin layer chromatography (Merck).
Procedure for the preparation of 2 from cholesterol
Silica gel (60–120 mesh, 1.0 g) was activated (150°C/1 mm Hg, 1 h) and mixed with cholesterol (0.38 g, 1.0 mmol) and NBS (recrystalized from hot water to have white crystals, 0.42 g, 5.0 mmol) taking in a dry mortar. The mixture was then pasted well to dust by a pestle, transferred to a round bottom flask, and was kept in stirring for 72 h. Next, Chloroform (50 mL) was poured, filtered, and the filtrate was washed with water (3×30 mL) and dried over Na2SO4. The solvent was evaporated and the residue was purified by column chromatography using petroleum ether as the eluent. Compound 2 appeared as pale greenish yellow gum (46%, 0.17 g, 0.46 mmol) with characteristic odor.
Compound 2
UV (Petroleum ether): λmax=277 nm, €=2360. IR (neat) (νmax/cm−1): 2951 (Ar–H str.), 2867 and 2929 (CH3 str.), 1462 (Arom. ring vib.), 1455 and 1380 (CH3 def.), 815 and 738 (Arom. H). 1H NMR (300 MHz, CDCl3): δ 0.68 (3H, s, CH3), 0.87 (3H, s, CH3), 0.85 (3H, s, CH3), 1.25 (3H, s, CH3), 2.19 (3H, s, CH3), 6.97 (1H, d, J =7.2 Hz, CH), 7.05 (1H, t, J=7.5 Hz, CH), 7.17 (1H, d, J=8.1 Hz, CH). 13C NMR (300 MHz, CDCl3): δ 11.9, 18.6, 19.8, 22.5, and 22.8 (5CH3), 23.8, 23.9, 26.8, and 27.2 (4CH2), 28.0 (CH), 28.3 and 29.7 (2CH2), 35.8 (CH), 36.2 (CH2), 37.8 (CH), 39.5 and 40.0 (2CH2), 42.6 (C), 44.4, 55.6, 56.3, 123.0, 125.2 and 127.1 (6CH), 135.1, 136.1 and 140.5 (3C). Anal. Calcd for C27H42 (366.34): C, 88.44; H 11.56%. Found: C, 88.51; H 11.47%. DART-MS, m/z (%): 366 (M+, 38), 365 (100), 364 (17), 363 (32), 361 (9), 353 (4), 351 (4).
Conclusion
A one-pot green approach has been developed to aromatize A-ring of cholesterol selectively using NBS. The simplicity of the reaction condition and a cost-effective reaction protocol is highly encouraging for the environmentally benign chemical transformations.
Acknowledgements
The authors are thankful to CDRI, Lucknow, India, for the mass spectrometry and elemental analysis. A.S. and J.D. thank UGC, New Delhi, and CSIR, New Delhi, respectively, for awarding Junior Research Fellowship.
References
- Marker , R.E. ; Kamm , O. ; Oakwood , T.S. ; Laucius , J.F. J. Am. Chem. Soc . 1936 , 58 , 1503 – 1504 .
- Inhoffen , H.H. ; Minlon , H. Naturwissenschaften 1938 , 26 , 756 .
- Inhoffen , H.H. ; Zuehlsdorff , G. Ber . 1941 , 74 , 604 – 616 .
- Clemo , G.R. ; Haworth , R.D. ; Walton , E. J. Chem Soc . 1930 , 1110 – 1115 .
- Inhoffen , H.H. Angew. Chem . 1940 , 53 , 471 – 475 .
- Wilds , A.L. ; Djerassi , C. J. Am. Chem. Soc . 1946 , 68 , 1712 – 1715 .
- Dreiding , A.S. ; Voltman , A. J. Am. Chem. Soc . 1954 , 20 , 537 – 539 .
- Morand , P. ; Lyall , J. Chem. Rev . 1968 , 68 , 85 – 124 .
- Romo , J. ; Djerassi , C. ; Rosenkranz , G. J. Org. Chem . 1950 , 15 , 896 – 900 .
- Romo , J. ; Rosenkranz , G. ; Djerassi , C. J. Org. Chem . 1950 , 15 , 1289 – 1292 .
- Hanson , J.R. ; Organ , T.D. J. Chem. Soc. C 1970 , 513 – 515 Hanson, J.R.; Organ, T.D. J. Chem. Soc. C 1970, 1313–1314 .
- Hanson , J.R. J. Chem. Soc. D 1971 , 1119 .
- Halcrow , M.A. ; Urbanos , F. ; Chaudret , B. Organometallics 1993 , 12 , 955 – 957 .
- Urbanos , F. ; Halcrow , M.A. ; Fernandez-Baeza , J. ; Dahan , F. ; Labroue , D. ; Chaudret , B. J. Am. Chem. Soc . 1993 , 115 , 3484 – 3493 .
- Alvarez , F.S. ; Ruiz , A.B. J.Org. Chem . 1965 , 30 , 2047 – 2049 .
- Kirdan , R.Y. ; Layne , D.S. J. Med. Chem . 1964 , 7 , 592 – 595 .
- Kaufmann , S. J. Org. Chem . 1966 , 31 , 2395 – 2397 .
- Anastasia , M. ; Ciuffreda , P. ; Puppo , M.D. ; Fiecchi , A. J. Chem. Soc. Perkin Trans . 1983 , 1 , 587 – 590 .
- Heller , M. ; Lenhard , R.H. ; Bernstein , S. J. Am. Chem. Soc . 1964 , 86 , 2309 – 2310 ; Heller, M.; Lenhard, R.H.; Bernstein, S. J. Am. Chem. Soc. 1967, 89, 1911–1918 .
- Libman , J.Q. ; Mazur , Y. J.Chem. Soc. D 1971 , 1146 – 1147 .
- Dryden , H.L. ; Webber , G.M. ; Wieczorek , J.J. J. Am. Chem. Soc . 1963 , 86 , 742 – 743 .
- Djerassi , C. ; Rosenkranz , G. ; Iriarte , J. ; Berlin , J. ; Romo , J. J. Am. Chem. Soc . 1951 , 73 , 1523 – 1527 .
- Tsuda , K. ; Ohki , E. ; Nozoe , S. J.Org. Chem . 1963 , 28 , 786 – 789 .
- Tsuda , D. ; Nozoe , S. ; Okada , Y. J. Org. Chem . 1963 , 28 , 789 – 792 .
- Tsuda , D. ; Nozoe , S. ; Tatezawa , T. ; Sharif , S.M. J. Org. Chem . 1963 , 28 , 795 – 798 .
- Suginome , H. ; Senboku , H. ; Yamada , S. Tetrahedon Lett . 1988 , 29 , 79 – 80 .
- Dodson , R.M. ; Muir , R.D. J. Am. Chem. Soc . 1961 , 83 , 4627 – 4631 ; Dodson, R.M.; Muir, R.D. J. Am. Chem. Soc. 1958, 80, 5004–5005 .
- Thomson , E.A. Jr. ; Siiteri , P.K. J. Biol. Chem . 1974 , 249 , 5373 – 5378 .
- Singh , K. ; Marshal , D.J. ; Vezina , C. Appl. Environ. Microbial . 1970 , 20 , 23 – 25 .
- Sih , C.J. ; Wang , K.C. J. Am. Chem. Soc . 1965 , 87 , 1387 – 1388 .
- Kautsky , M.P. ; Hagerman , D.D. Steroids 1976 , 28 , 247 – 259 .
- Moortgy , K.B. ; Meigs , R.A. Biochim. Biophys. Acta 1978 , 528 , 222 – 229 .
- Payne , A.H. ; Hales , D.B. Endocr. Rev . 2004 , 25 , 947 – 970 .
- Caspi , E. ; Arunachalam , T. ; Nelson , P.A. J. Am. Chem. Soc . 1986 , 108 , 1847 – 1852 .
- Sondheimer , F. ; Neumann , F. ; Ringold , H.J. ; Rosenkranz , G. J. Am. Chem. Soc . 1954 , 76 , 2230 – 2233 .