Abstract
Various 2,6-dibenzylidene cycloalkanones were readily prepared in a few minutes with good yields by simply mixing aromatic aldehydes with cycloalkanones in the presence of catalytic amounts of p-toluenesulfonic acid as a solid heterogeneous catalyst under solvent-free condition.
Introduction
Cross-Aldol condensation of aromatic aldehydes with cyclicketones is an important synthetic reaction for the preparation of α,α′-bis(substituted benzylidene) cycloalkanones. These benzylidene derivatives are intermediates of various pharmaceuticals, agrochemicals, and perfumes Citation1. They are frequently used for the synthesis of biological activities such as HIV-1 integrase inhibitory Citation2, bioactive pyrimidene compounds Citation3. They also find applications in the preparation of nonlinear optical materials Citation4 and liquid-crystalline polymers Citation5.
The typical methods for the preparation of α,α′-bis(substituted benzylidene) cycloalkanones generally involve the cross-aldol condensation of cycloalkanone with aldehydes under strong acids or bases Citation6. However, this process suffers from reverse and side reactions resulting in low yields of the products.
Different complexes of metal(II) ions have also been used as catalysts but the yields were not satisfactory Citation7. Various other reagents such as BMPTO Citation8, KF–Al2O3 Citation9, FeCl3 Citation10, Yb(OTf)3 Citation11, Cu(OTf)2 Citation12, I2 Citation13, and animal bones meal Citation14 are known to catalyze the reaction. However, in most cases the yields are good at high temperatures and some of the reagents require longer reaction times and tedious purification procedures. Thus, there is a certain need for the development of an alternative route for the production of α,α′-bis(substituted benzylidene) cycloalkanones, which surpasses those limitations.
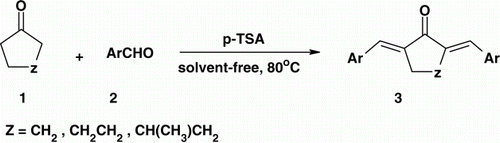
During the course of our systematic studies directed toward the development of environmentally friendly procedures for several important organic transformations Citation15–18 herein we describe an efficient method for the synthesis of α,α'-bis(substituted benzylidene) cycloalkanones through reaction of aldehydes with cycloalkanones in the presence of catalytic amounts of p-toluenesulfonic acid under solvent-free condition ().
In the organic synthesis and reactions, increasing attention is being focused on green chemistry using environmentally benign reagents and conditions, particularly solvent-free procedures, which often lead to clean, eco-friendly and highly efficient procedures involving simplified work-ups. The use of solid acid catalysts has gained a vast importance in organic synthesis due to their several advantages such as operationally simplicity, no toxicity, reusability, low cost, and easy isolation after completion of reaction. In recent years p-toluenesulfonic acid (p-TSA) has been considered as an efficient, inexpensive, and readily available catalyst for several organic transformations Citation19–21. Because of the application of this catalyst for development of useful synthetic methodologies we have observed that this is very suitable to catalyze the cross-Aldol condensation of aromatic aldehydes with cyclic ketones at 80°C under solvent–free conditions.
Results and discussion
Firstly, reaction of benzaldehyde and cyclohexanone was chosen as the model reaction to detect whether the use of p-TSA was efficient and to investigate the optimized conditions. The best result was achieved by carrying out the reaction in the presence of 20 mol% of p-TSA with 2:1 mole ratios of benzaldehyde and cyclohexanone at 80°C for 20 min (, Entry 1). To establish the generality and scope of p-TSA as a catalyst for this synthesis, various aldehydes (2) and cycloalkanones (1), was reacted following the optimized method to prepare a series of α,α′-bis(substituted benzylidene) cycloalkanones. Several examples illustrating this novel and general method for the synthesis of 3a-t are summarized in . In all these cases, the corresponding products were obtained in low reaction times with good yields.
Table 1. Preparation of α,α′-bis(substituted benzylidene) cycloalkanone catalyzed by p-TSA at 80°C under solvent-free conditions.
Aromatic aldehydes containing both electron donating and withdrawing groups underwent the conversion smoothly and gave the products in good to excellent yields (). So it is concluded that the electronic nature of the substituents has no significant effect on this reaction. However, the reaction of p-cholorobenzaldehyde with cyclohexanone (Entry 2) gives a relatively high yield, when compared with other aromatic aldehydes.
All the reactions were free from by-products, which are generally found in classical reactions conditions. The structure of the products was deduced from their IR, 1H NMR, and 13C NMR.
To illustrate the need of p-TSA for these reactions an experiment was conducted in the absence of p-TSA. The yield in this case was traced after 24 h. Obviously, p-TSA is an important component of the reaction.
In order to show the merits of p-TSA over other catalysts reported in the literature, results with this catalyst were compared with other catalysts utilized for the synthesis of α,α'-bis(substituted benzylidene) cycloalkanones. From , it can be seen that p-TSA appears to promote the reaction more effectively than a number of other catalysts, particularly in terms of the time and yield required to complete the reaction. This method offers some advantages in terms of low reaction times, simplicity of performance, solvent-free condition, low cost, and it follows along the line of green chemistry. The catalyst is readily available and inexpensive and can conveniently be handled and removed from the reaction mixture.
Table 2. Comparison of results using p-TSA with other heterogeneous catalyst for synthesis of α,α'-bis(substituted benzylidene) cycloalkanones.
In conclusion this paper describes efficient method for cross-Aldol condensation of aromatic aldehydes with cyclic ketones to form α,α′-bis(substituted benzylidene) cycloalkanones using p-TSA as a solid heterogeneous catalyst at 80°C under solvent-free conditions. The mild reaction conditions, shorter reaction times, high yields, and application of an inexpensive and readily available catalyst are the great advantages of the method.
Experimental
General procedure for the preparation of α,α′-bis(substituted benzylidene) cycloalkanones
A mixture of cycloalkanone 1 (5 mmol), aldehyde 2 (10 mmol), and p-TSA (1 mmol) was added to a round-bottomed flask equipped with a reflux condenser. The mixture was stirred at 80°C for an indicated time to complete the reaction monitored by thin-layer chromatography (TLC). After completion of the reaction, the reaction mixture was cooled to room temperature. Water (10 mL) was added and the mixture was stirred for 10 min in order to solve the catalyst. The obtained solid was collected by filtration to give, in many cases, the pure α,α′-bis(substituted benzylidene) cycloalkanones. In some cases, the aforementioned residue was purified by crystallization (EtOAc-EtOH) to afford pure products.
Selected spectral data
2,6-Dibenzylidene cyclohexanone (3a)
IR (KBr): 3072, 3023, 1658, 1570, 1445, 1271, 1415, 1271, 1145, 770 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.81 (s, 2 H, =CH), 7.46–7.24 (m, 10H, ArH), 2.92 (t, 4H, J =5.6 Hz, CH2), 1.80–1.74 (m, 2H, CH2).
2,6-Di(4-chlorobenzylidene)cyclohexanone (3b)
IR (KBr): 2927, 1664, 1608, 1579, 1270, 833 cm−1; 1H NMR (400 MHz, CDCl3): δ=7.73 (s, 2 H, =CH), 7.41–7.36 (m, 8H, ArH), 2.90 (t, 4H, J=6.0 Hz, CH2), 1.83–1.79 (m, 2H, CH2).
2,6-Di(3-nitrobenzylidene)cyclohexanone (3c)
IR (KBr): 3108, 2928, 1669, 1591, 1514, 1490, 1343, 1267, 856 cm−1; 1H NMR (400 MHz, CDCl3): δ=8.33 (s, 2 H, =CH), 8.22 (d, 2H, J=8.0 Hz, ArH), 7.81–7.60 (m, 6H, ArH), 2.98 (t, 4 H, J=5.6Hz, CH2), 1.90–1.84 (m, 2H, CH2).
2,6-Di(3-flourobenzylidene)cyclohexanone (3f)
IR (KBr): 2943, 1662, 1581, 1437, 1169, 1145,783 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.76 (s, 2 H, =CH), 7.43–7.38 (m, 2H, ArH), 7.25 (d, 2H, J =8.0 Hz, ArH), 7.19 (dd, 2H, J=10 Hz, J =2 Hz, ArH) 7.10–7.05 (m, 2H, ArH), 2.96–2.93 (m, 4 H, CH2), 1.87–1.81 (m, 2H, CH2).
2,5-Dibenzylidenecyclopentanone (3i)
IR (KBr): 3053, 3018, 1690, 1626, 1602 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.60–7.58 (m, 6H, ArH, and =CH), 7.45–7.36 (m, 6H, ArH), 3.13 (s, 4H, CH2).
2,5-Di(4-methoxybenzylidene)cyclopentanone (3k)
IR (KBr): 3080, 2851, 1684, 1590 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.59 (d, 4H, J=8.0 Hz, ArH), 7.58 (s, 2H, =CH), 6.98 (d, 4H, J =8.0 Hz, ArH), 3.88 (s, 6H, OCH3), 3.07(s, 4H, CH2).
2,5-Di(4-nitrobenzylidene)cyclopentanone (3m)
IR (KBr): 3105, 2843, 1706, 1605, 1522 cm−1; 1H NMR (400 MHz, CDCl3): δ = 8.32–8.30 (m, 4H, ArH), 7.76–7.65 (m, 6H, ArH, and =CH2), 3.20 (s, 4H, CH2)
2,5-Di(3-flourobenzylidene)cyclopentanone (3n)
IR (KBr): 2943, 1668, 1601, 1441, 1169, 1210,778 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.56 (s, 2 H, =CH), 7.46–7.38 (m, 4H, ArH), 7.33–7.29 (m, 2H, ArH) 7.14–7.10 (m, 2H, ArH), 3.14(s, 4H, CH2).
2,6-Di(3-nitrobenzylidene)4-methylcyclohexanone (3q)
IR(KBr): 2956, 1667, 1608, 1526, 1348, 1196, 1147, 891, 820, 748, 674 cm−1; 1H NMR (400 MHz, CDCl3): δ = 8.32 (s, 2H, =CH), 8.25–8.23 (m, 2H, ArH), 7.83 (s, 2 H, ArH), 7.78 (d, 2H, J =8.0 Hz, ArH), 7.64 (t, 2H, J =8.0 Hz, ArH), 3.05 (dd, 2H, J =3.6 Hz, J =3.6 Hz, CH2), 2.66–2.58 (m, 2H, CH2), 2.02–1.93 (m, 2H, CH), 1.13 (d, 3H, J =6.4 Hz, CH3). Anal. calcd. for C21H18N2O5 (378.38): C, 66.66; H, 4.79; N, 7.40%. Found: C, 66.56; H, 4.65. N, 7.34%.
2,6-Di(3-flourobenzylidene)4-methylcyclohexanone (3r)
IR(KBr): 2927, 1663, 1612, 1584, 1431, 1213, 1152, 1016, 784, 682 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.76 (s, 2 H, =CH), 7.43–7.38 (m, 2H, ArH),7.29–7.25 (m, 2H, ArH), 7.19–7.17 (m, 2H, ArH), 7.10–7.05 (m, 2H, ArH), 3.06 (dd, 2H, J =3.6 Hz, J =3.6 Hz, CH2) 2.57–2.50 (m, 2H, CH2), 1.97–1.88 (m, 1H, CH), 1.12 (d, 3H, J =6.8 Hz, CH3). Anal. calcd. for C21H18F2O (324.36): C, 77.76; H, 5.59%. Found: C, 77.61; H, 5.51%.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Islamic Azad University, Firoozabad Branch.
References
- Song , D. ; Chen , Y. ; Wang , R. ; Liu , C. ; Jiang , H. ; Luo , G. Prep. Biochem. Biotechol . 2009 , 39 , 201 – 207 .
- Costi , R. ; Santo , R.D. ; Artico , M. ; Massa , S. ; Rango , R. ; Laddo , R. ; Colla , M.L. ; Tramontano , E. ; Colla , P.L. ; Pani , A. Bioorg. Med. Chem . 2004 , 12 , 199 – 215 .
- Deli , J. ; Lorand , T. ; Szabo , D. ; Fddesi , A. Pharmazie . 1984 , 39 , 539 – 540 .
- Kawamata , J. ; Inoue , K. ; Inabe , T. ; Kiguchi , M. ; Kato , M. ; Taniguchi , Y. Chem. Phys. Lett . 1996 , 249 , 29 – 34 .
- Gangadhara , K.K. Polymer 1995 , 36 , 1903 – 1910 .
- Hathaway , B.A. J. Chem. Educ . 1987 , 64 , 367 – 368 .
- Irie , K. ; Watanabe , K. Bull. Chem. Soc. Jpn . 1980 , 53 , 1366 – 1371 .
- Zheng , M. ; Wang , L. ; Shao , J. ; Zhong , Q. Synth. Commun . 1997 , 27 , 351 – 354 .
- Yadav , J.S. ; Reddy , B.V.S. ; Nagaraju , A. ; Sarma , J.A.R.P. Synth. Commun . 2002 , 32 , 893 – 896 .
- Zhang , X.Y. ; Fan , X.S. ; Niu , H.Y. ; Wang , J.J. Green Chem . 2003 , 5 , 267 – 269 .
- Wang , L.M. ; Sheng , J. ; Tian , H. ; Han , J.W. ; Fan , Z.Y. ; Qian , C.T. Synthesis 2004 , 3060 – 3064 .
- Li , J. ; Su , W. ; Li , N. Synth. Commun . 2005 , 35 , 3037 – 3043 .
- Das , B. ; Thirupathi , P. ; Mahender , I. ; Redday , K.R. J. Mol. Catal. A: Chem . 2006 , 247 , 182 – 185 .
- Riadi , Y. ; Mamouni , R. ; Azzalou , R. ; Boulahjar , R. ; Abrouki , Y. ; El Haddad , M. ; Routier , S. ; Guillaumet , G. ; Lazar , S. Tetrahedron Lett . 2010 , 51 , 6715 – 6717 .
- Karimi-Jaberi , Z. ; Amiri , M. Heteroatom Chem . 2010 , 21 , 96 – 98 .
- Karimi-Jaberi , Z. ; Keshavarzi , M. Chin. Chem. Lett . 2010 , 21 , 547 – 549 .
- Karimi-Jaberi , Z. ; Hashemi , M.M. Monatsch. Chem . 2008 , 139 , 605 – 608 .
- Karimi-Jaberi , Z. ; Amiri , M. ; Sadeghi , N. Synth. Commun. . 2010 , 40 , 2948 – 2953 .
- Longhi , K. ; Moreira , N. ; Morzari , M.R.B. ; Floss , V.M. ; Bonacorso , H.G. ; Zanatta , N. ; Martins , M.A.P. Tetrahedron Lett . 2010 , 51 , 3193 – 3196 .
- Huang , P.-J.J. ; Cameron , T.S. ; Jha , A. Tetrahedron Lett . 2009 , 50 , 51 – 54 .
- Mogilaiah , K. ; Chowdary , D.S. ; Redday , P.R. ; Redday N.V. Synth. Commun . 2003 , 33 , 127 – 131 .
- Tao , X.C. ; Liu , R.Z. ; Meng , Q.H. ; Zhao , Y.Y. ; Zhou , Y.B. ; Huang , J.L. J. Mol. Catal. A: Chem . 2005 , 225 , 239 – 243 .
- Li , J. ; Yang , W. ; Chen , G. ; Li , T. Synth. Commun . 2003 , 33 , 2619 – 2625 .
- Salehi , P. ; Dabiri , M. ; Zolfigol , M.A. ; Bodaghi Fard , M. J. Braz. Chem. Soc . 2004 , 15 , 773 – 776 .
- Wan , Y. ; Chen , X.M. ; Pang , L.L. ; Ma , R. ; Yue , C.H. ; Yuan , R. ; Lin , W. ; Yin , W. ; Bo , R.C. ; Wu , H. Synth. Commun . 2010 , 40 , 2320 – 2328 .
- Lin , P. ; Li , B. ; Li , J. ; Wang , H. ; Bian , X. ; Wang , X. Catal. Lett . 2011 , 141 , 459 – 466 .
- Bigdeli , M.A. ; Mahdavinia , G.H. ; Jafari , S. ; Hazarkhani , H. Catal. Commun . 2007 , 8 , 2229 – 2231 .