Abstract
Crossed aldol condensation of aromatic aldehydes with cyclic ketones in the presence of catalytic amount of NH4H2PO4/SiO2 (as a safe, green, and cheap heterogeneous catalyst) under solvent-free condition afforded α,ά-bis(substituted-benzylidene) cycloalkanones in high yields. This method is general with respect to all types of aromatic aldehydes and is an eco-friendly procedure. And the catalyst is easily prepared, stable, reusable, and efficient under the reaction conditions.
Introduction
Cross-aldol condensation of aromatic aldehydes with cyclic ketones is an important protocol for the synthesis of α,ά-bis(substituted-benzylidene)cycloalkanones, which are very important precursors to potentially bioactive pyrimidine derivates Citation1, intermediates for agrochemical, pharmaceuticals and perfumes Citation2, new organic material for nonlinear optical applications Citation3, cytotoxic analogous Citation4, bis-spiropyrrolidines Citation5, and the units of liquid crystalline polymers Citation6. Usually, this condensation process is catalyzed by strong acid or base, however, suffers from side reactions (such as self-condensation of ketones and aldehydes) giving the corresponding products in low yields Citation7 Citation8. Therefore, numerous studies in the literature to improve the performance of the reaction have existed; the main progress was the following: (1) The aldol reaction was catalyzed by organometallic complexes but the yields were not satisfactory Citation9 or required long reaction time Citation10, (2) Lewis acid such as RuCl3 Citation11, SmI3 Citation12, BF3Et2O Citation13 Citation14, FeCl3 6H2O Citation15, Mg(HSO4)2 Citation16, KHSO4 Citation17, Yb(OTf)3 Citation18, Cu(II) trifluoroacetate Citation19, InCl3 4H2O Citation20, LiClO4 Citation21, NKC-9 (polymer-supported sulfonic acid) Citation22, SiO2–Pr–SO3H Citation23 were used to promote the aldol reaction, and (3) I2 Citation24, TMSCl/NaI Citation25, LiOH Citation26, KF/Al2O3 Citation27, [(Me3Si)2N]3Ln(µ-Cl)Li(THF)3 Citation28, Silica Chloride Citation29, BMPTO(bis(p-ethoxyphenyl)telluroxide) Citation30, TCT(2,4,6-trichloro[1,3,5]triazine) Citation31 NaOH in micellar medium Citation32, KOH Citation33, Na-HAP Citation34, and animal bone meal Citation35 were also found to accelerate this type of reaction. However, the use of toxic reagents, long reaction time, low yields, formation of a mixture of products, and tedious separation procedures is among the drawbacks of the reported methods. It is therefore important to find a more convenient method for the preparation of these compounds.
Silica gel and silica-supported reagents has been used as catalyst in organic synthesis because it is easily available, inexpensive, and nontoxic. Use of such a heterogeneous catalyst benefits several potential catalyst reuses and waste production minimizations Citation36–38.
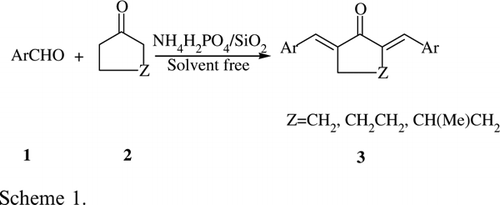
Among of many silica-supported catalysts employed, NH4H2PO4/SiO2, one of the solid supported catalysts has already been prepared by our research group Citation39 and approved to be a potential green catalyst with several advantages such as low toxicity, low cost, ease of handling, and high catalytic activity. In continuation of our work to develop new and eco-friendly synthetic methodologies, herein we report a novel, green, facile, and efficient one-pot method for the synthesis of α,ά-bis(substituted-benzylidene) cycloalkanones catalyzed by NH4H2PO4/SiO2 under solvent-free condition ().
Results and discussion
Initially, the reaction was performed by reacting cyclohexanone (1 equiv) and benzaldehyde (2 equiv) in the presence of 0.05 g of NH4H2PO4/SiO2 as a catalyst under solvent-free condition at 80°C. Under these conditions, only a trace amount of product was obtained after 150 min. The conditions for this transformation were optimized and the results are shown in . No product was obtained in the absence of the catalyst (, entry 1) even when the reaction time was extended to 180 min., thus demonstrating the importance of NH4H2PO4/SiO2. The amount of the catalyst required for the transformation was investigated by the use of 0.05–0.15 g. of catalyst. Under these conditions, the yields were in a range of 10–90% (). The use of 0.15 g of NH4H2PO4/SiO2 gave the best result (, entry 9). The reaction worked well with electron-withdrawing (NO2, Cl) as well as electron-donating (Me, MeO, N(CH3)2) groups, giving various α,ά-bis(substituted-benzylidene) cycloalkanones in 85–94% yields. As it is shown in , the method is general and encompasses a variety of aromatic aldehydes with excellent yields. The reaction worked with aliphatic aldehydes but did not react well.
Table 1. Reaction of benzaldehyde and cyclohexanone under solvent-free conditions.
Table 2. Preparation of α,ά-bis(substituted benzylidene)cycloalkanones catalyzed by NH4H2PO4/SiO2 at 110°C under solvent-free conditions.
Experimental
Preparation of NH4H2PO4/SiO2
The catalyst was prepared by mixing silica gel (1.5 g, Merck grade 60, 230–400 mesh) with a solution of NH4H2PO4 (0.6 g, 5 mmol) in distilled water (10 mL). The resulting mixture was stirred for 30 min to absorb NH4H2PO4 on surface of silica gel (). After removal of water in a rotary evaporator, the solid powder was dried at 120°C for 2–3 h under reduced pressure. The drying temperature was maintained below the decomposition temperature of the salt.
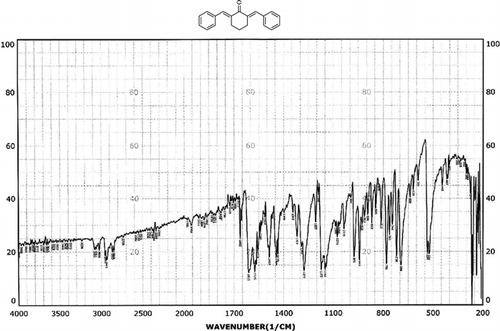
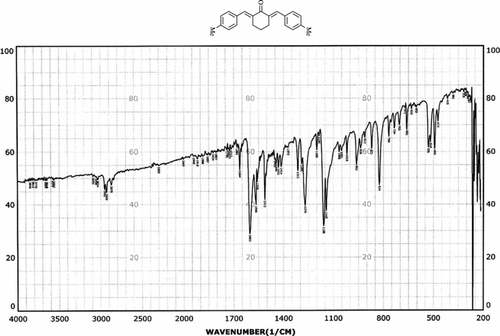
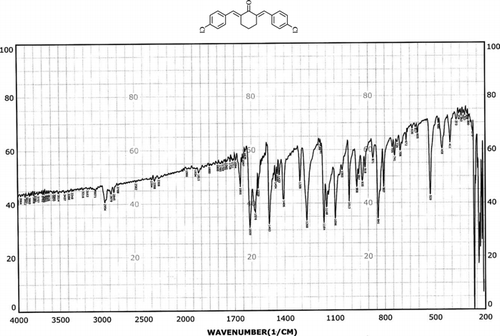
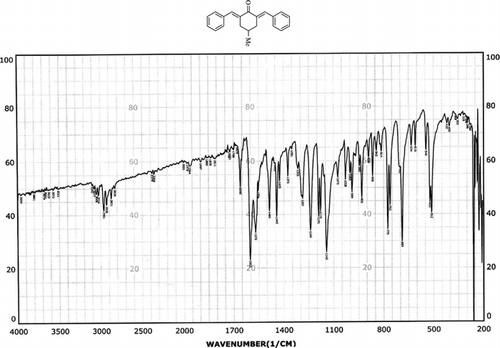
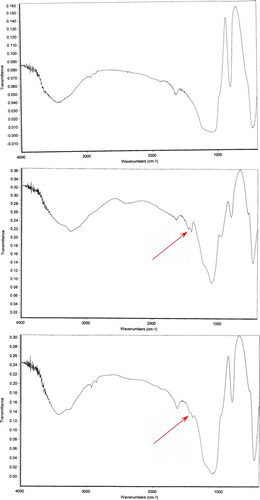
Infrared spectras of silica gel and catalyst, before and after using it, were shown in . Frequency comparison of IR spectras show the appearance of absorption in region 1400 cm−1. This absorption is due to the P=O stretching vibrations of –OPO3H2 groups presence that was shown in .
Synthesis of α,ά-bis(substituted-benzylidene) cycloalkanones: general procedure
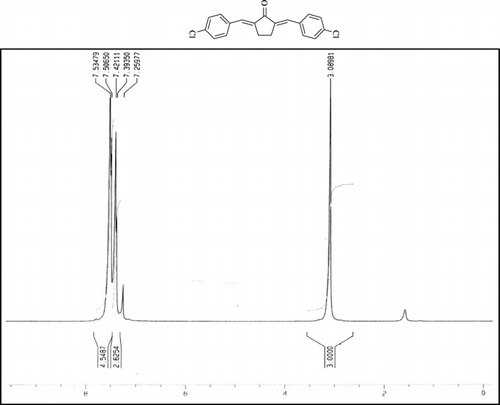
A mixture of aromatic aldehyde (2 mmol), cyclic ketones (1 mmol), and NH4H2PO4/SiO2 (0.15 g) was stirred for 2 min at room temperature and then the temperature was raised to 110°C and maintained for the appropriate time (). After completion of the reaction (monitored by TLC), the reaction mixture was diluted with hot ethanol and the catalyst was filtrated off, the residue was kept at room temperature and the resulting crystal product was collected by filtration and washed with ethanol. The product was found to be pure and no further purification was necessary.
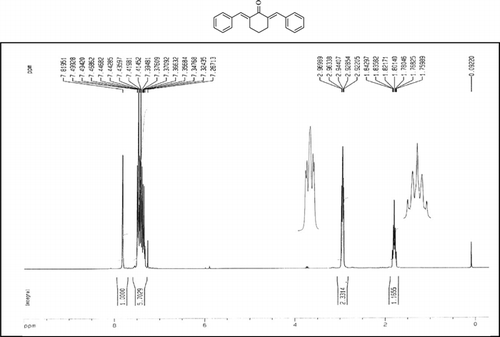
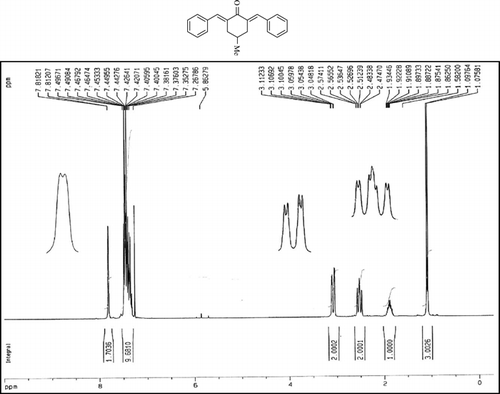
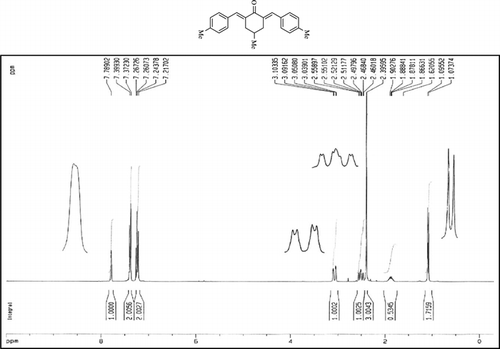
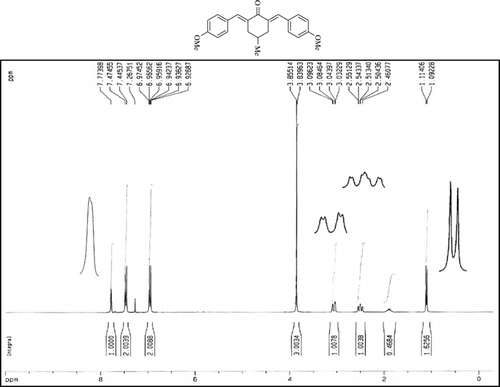
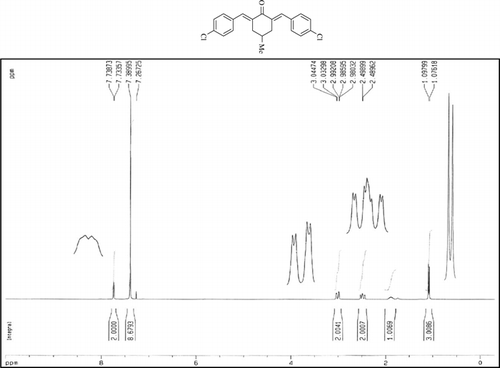
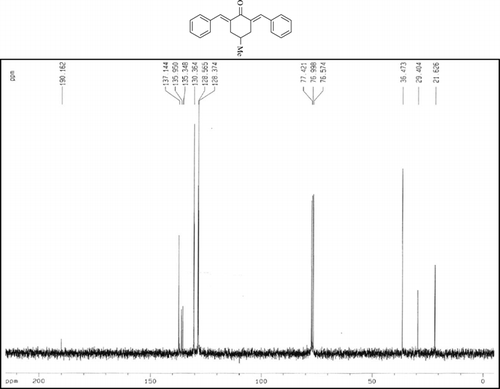
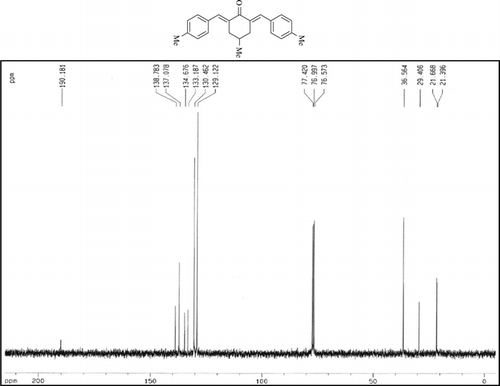
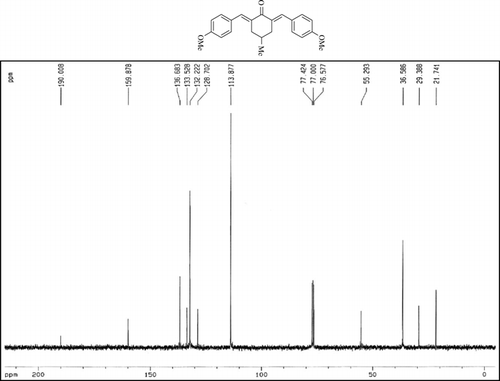
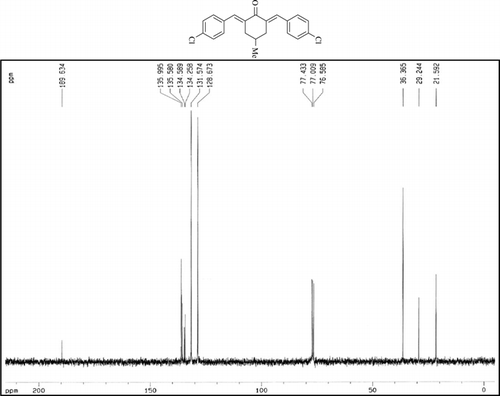
The 1H-NMR data of some compounds are given below:
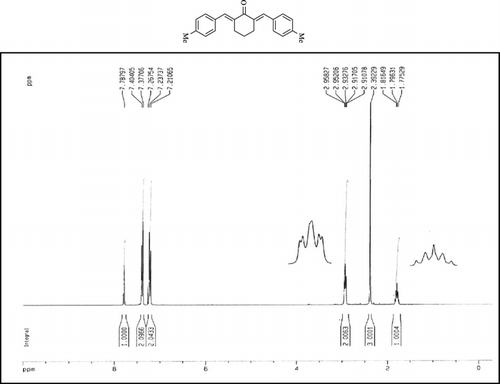
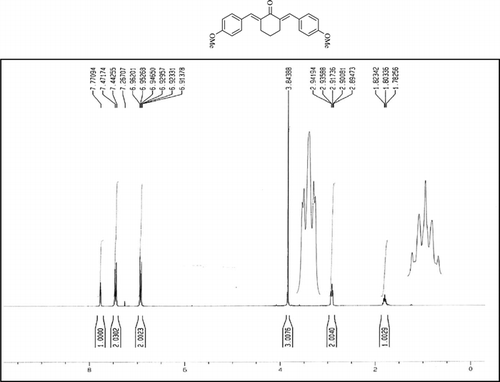
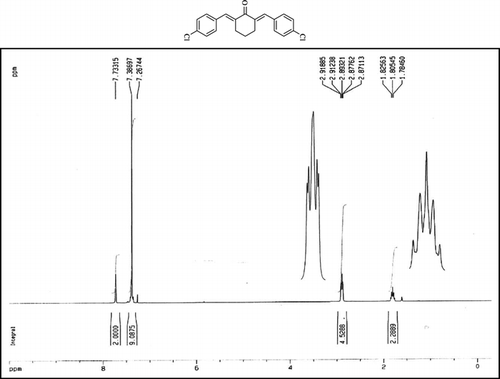
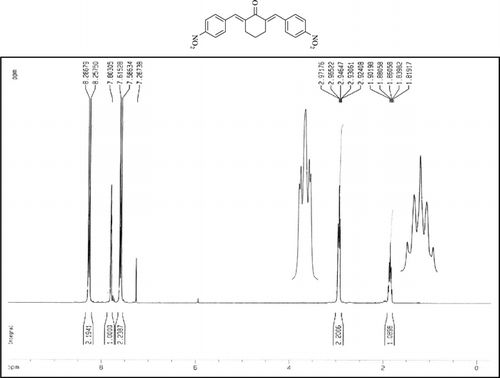
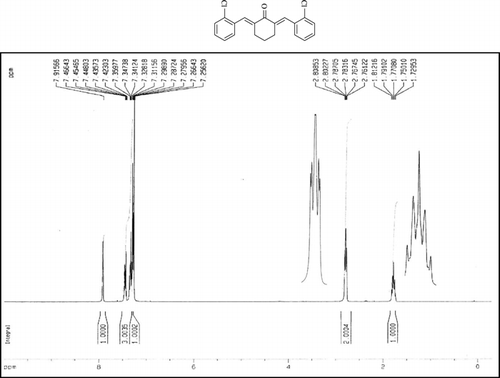
Compound 3q. 1H-NMR (300 MHz, CDCl3): δ 7.81 (dbr, 2H, J = 1.6 Hz), 7.32–7.49 (10H, Ar), 3.10 (ddbr, 2H, J = 3.5, 15.8 Hz), 2.52 (ddd, 2H, J = 2.6, 11.4, 15.8 Hz), 1.89 (m, 1H), and 1.10 (d, 3H, J = 6 Hz).
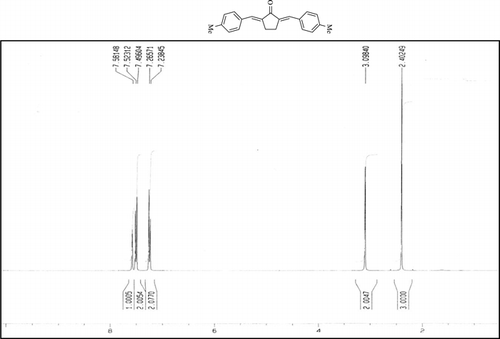
Compound 3r. 1H-NMR (300 MHz, CDCl3): δ 7.79 (dbr, 2H, J = 1.6 Hz), 7.21–7.39 (8H, Ar), 3.10 (ddbr, 2H, J = 3.5, 15.7 Hz), 2.50 (ddd, 2H, J =2.4, 11.3, 15.7 Hz), 2.39 (s, 3H), 1.87 (m, 1H), and 1.08 (d, 3H, J = 6.3 Hz).
Compound 3s.1H-NMR (300 MHz, CDCl3): δ 7.77 (dbr, 2H, J = 1.6 Hz), 6.92–7.47 (8H, Ar), 3.85 (s, 3H), 3.06 (ddbr, 2H, J =3.5, 15.7 Hz), 2.50 (ddd, 2H, J =2.4, 11.3, 15.7 Hz), 1.88 (m, 1H), and 1.10 (d, 3H, J = 6.5 Hz).
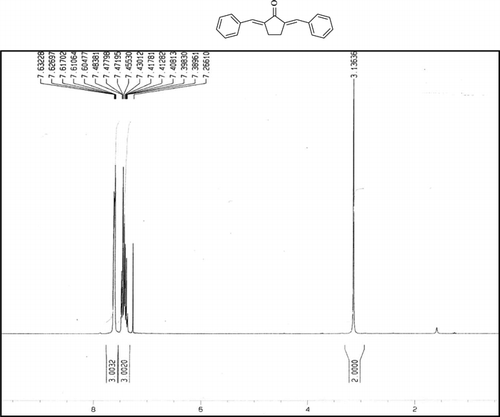
Compound 3t. 1H-NMR (300 MHz, CDCl3): δ 7.73 (dbr, 2H, J = 1.6 Hz), 7.38 (8H, Ar), 2.99 (ddbr, 2H, J = 3.5, 15.7 Hz), 2.48 (ddd, 2H, J = 2.6, 11.2, 15.7 Hz), 1.88 (m, 1H), and 1.08 (d, 3H, J = 6.5 Hz).
Reusability of catalyst
Next, we investigated the reusability and recycling of NH4H2PO4/SiO2. A mixture of p-cholorobenzaldehyde, cyclopentanone, and H4H2PO4/SiO2 was stirred at 110°C in solvent-free condition. After completion of the reaction, the reaction mixture was diluted with hot ethanol and the catalyst was separated by simple filtration and recovered NH4H2PO4/SiO2 was reused in subsequent reactions without significant decrease in activity even after five runs ().
Table 3. The recycling of NH4H2PO4/SiO2 in synthesis of 3d.
Conclusions
We described herein an ammonium dihydrogen phosphate adsorbed on silica gel (NH4H2PO4/SiO2) catalyzed highly efficient, one-pot, green protocol for the synthesis of α,ά-bis(substituted-benzylidene) cycloalkanones by the condensation of aromatic aldehydes and cyclic ketones under solvent-free condition in very good to excellent yields. The present methodology offers several advantages such as simple procedure, low cost, easy work-up, short reaction time, and milder condition.
Acknowledgements
We thank the faculty of chemistry of K.N. Toosi University of Technology and Islamic Azad University-Marvdasht Branch for supporting this work.
References
- Deli , J. ; Lorand , T. ; Szabo , D. ; Foldesi , A. Pharmazie . 1984 , 39 , 539 – 540 .
- Ogawa , M. ; Ishii , Y. ; Nakano , T. ; Irifune , S. Jpn. Kohai Tokkyo . 1988 , JP 63192446 A2; Ogawa, M.; Ishii, Y.; Nakano, T.; Irifune, S. Chem. Abstr. 1988, 63, 238034 .
- Kawamata , J. ; Inoue , K. ; Inabe , T. ; Kiguchi , M. ; Kato , M. ; Taniguchi , Y. Chem. Phys. Lett . 1996 , 249 , 29 – 34 .
- Dimmock , J.R.A. ; Padmanilayam , M.P. ; Zello , G.A. ; Nienaber , K.H. ; Allen , T.M. ; Santos , C.L. ; De Clercq , E. ; Balzarini , J. ; Manavathu , E.K. ; Stables , J.P. Eur. J. Med. Chem . 2003 , 38 , 169 – 177 .
- Raj , A.A. ; Raghunathan , R. ; Sridevi Kumari , M.R. ; Raman , N. Bioorg. Med. Chem . 2003 , 11 , 407 – 419 .
- Gangadhara , K.K. Polymer . 1995 , 36 , 1903 – 1910 .
- Hathaway , B.A. J. Chem. Edu . 1987 , 64 , 367 – 368 .
- Nakano , T. ; Irifune , S. ; Umano , S. ; Inada , A. ; Ishii , Y. ; Ogawa , M. J. Org. Chem . 1987 , 52 , 2239 – 2244 .
- Irie , K. ; Watanabe , K. Bull. Chem. Soc. Jpn . 1980 , 53 , 1366 – 1371 .
- Nakano , T. ; Migita , T. Chem. Lett . 1993 , 22 , 2157 – 2158 .
- Iranpoor , N. ; Kazemi , F. Tetrahedron . 1998 , 54 , 9475 – 9480 .
- Bao , W.L. ; Zhang , Y.M. ; Taokai , Y. Synth. Commun . 1996 , 26 , 503 – 507 .
- Huang , D.F. ; Wang , J.X. ; Hu , Y.L. Chin. Chem. Lett . 2003 , 14 , 333 – 334 .
- Shinji , N. ; Aki , M. ; Fumiko , I. ; Takayoshi , Y. ; Hiroshi , S. ; Kiyoshi , S. J. Chem. Soc. Chem. Commun . 1990 , 21 , 1538 – 1539 .
- Zhang , X.Y. ; Fan , X.S. ; Niu , H.Y. ; Wang , J.J. Green Chem . 2003 , 5 , 267 – 269 .
- Salehi , P. ; Khodaei , M.M. ; Zolfigol , M.A. ; Keyvan , A. Monatsh. Chem . 2002 , 133 , 1291 – 1295 .
- Cai , X.H. ; Xie , B. ; Guo , H. Chem. Pap . 2006 , 60 , 318 – 320 .
- Wang , L.M. ; Sheng , J. ; Tian , H. ; Han , J. ; Fan , Z. ; Qian , C. Synthesis 2004 , 3060 – 3064 .
- Song , D. ; Chen , Y. ; Wang , R. ; Liu , C. ; Jiang , H. ; Luo , G. Prep. Biochem. & Biotech . 2009 , 39 , 201 – 207 .
- Yang , L. ; Lu , J. ; Bai , Y.J. Chin. J. Org. Chem . 2003 , 23 , 659 – 661 .
- Arnold , A. ; Markert , M. ; Mahrwald , R. Synthesis 2006 , 1099 – 1102 .
- An , L.T. ; Zou , J.P. ; Zhang , L.L. Catal. Commun . 2008 , 9 , 349 – 354 .
- Ziarani , G.M. ; Badiei , A. ; Abbasi , A. ; Farahani , Z. Chin. J. Chem . 2009 , 27 , 1537 – 1542 .
- Das , B. ; Thirupathi , P. ; Mahender , I. ; Reddy , K.R. J. Mol. Catal. A: Chem . 2006 , 247 , 182 – 185 .
- Sabitha , G. ; Reddy , G.S.K. ; Reddy , K.B. ; Yadav , J.S. Synthesis 2004 , 263 – 266 .
- Bhagat , S. ; Sharma , R. ; Chakraborti , A.K. J. Mol. Cata. A: Chem . 2006 , 260 , 235 – 240 .
- Yadav , J.S. ; Reddy , B.V.S. ; Nagaraju , A. ; Sarma , J.A.R.P. Synth. Commun . 2002 , 32 , 893 – 896 .
- Zhang , L. ; Wang , S. ; Sheng , E. ; Zhou , S. Green Chem . 2005 , 7 , 683 – 686 .
- Hazarkhani , H. ; Kumar , P. ; Kondiram , K.S. ; Shafi , G. ; Ikhlas , M. Synth. Commun . 2010 , 40 , 2887 – 2896 .
- Zheng , M. ; Wang , L. ; Shao , J. ; Zhong , Q. Synth. Commun . 1997 , 7 , 351 – 354 .
- Bigdeli , M.A. ; Mahdavinia , G.H. ; Jafari , S. ; Hazarkhani , H. Catal. Commun . 2007 , 8 , 2229 – 2231 .
- Shrikhande , J.J. ; Gawande , M.B. ; Jayaram , R.V. Catal. Commun . 2008 , 9 , 1010 – 1016 .
- Singh , N. ; Pandey , J. ; Yadav , A. ; Chaturvedi , V. ; Bhatnagar , S. ; Gaikwad , A.N. ; Sinha , S.K. ; Kumar , A. ; Shukla , P.K. ; Tripathi , R.P. Eur. J. Med. Chem . 2009 , 44 , 1705 – 1709 .
- Solhy , A. ; Amer , W. ; Karkouri , M. ; Tahir , R. ; Bouari , A.E. ; Fihri , A. ; Bousmina , M. ; Zahouily , M. J. Mol. Catal. A: Chem . 2011 , 336 , 8 – 15 .
- Riadi , Y. ; Mamouni , R. ; Azzalou , R. ; Boulahjar , R. ; Abrouki , Y. ; El Haddad , M. ; Routier , S. ; Guillaumet , G. ; Lazar , S. Tetrahedron Lett . 2010 , 51 , 6715 – 6717 .
- Wu , H. ; Lin , W. ; Wan , Y. ; Xin , H.-Q. ; Shi , D.-Q. ; Shi , Y.-H. ; Yuan , R. ; Bo , R.-C. ; Yin , W. J. Comb. Chem . 2010 , 12 , 31 – 34 and references cited therein .
- Lancaster , M. Green Chemistry: An Introductory Text ; Cambridge : Royal Society of Chemistry , 2002 ; 87 – 96 .
- Matlack , A.S. Introduction to Green Chemistry ; Marcel Dekker : New York-Basel , 2001 ; 114 , 191 .
- Mahdavinia , G.H. ; Rostamizadeh , Sh. ; Amani , A.M. ; Emdadi , Z. Ultrason. Sonchem . 2008 , 16 , 7 – 10 .
Appendix A: Supporting information for