Abstract
An expeditious synthesis of 4H-chromenes has been achieved by three-component one-pot condensation of 1,3-diketone, aldehyde, and malononitrile using Ba(OTf)2 as catalyst in PEG-water at room temperature. Results from various reaction media and different metal triflates as catalysts show that choice of proper catalyst and the solvent system play a key role in the synthesis of 4H-chromene-3-carbonitrile derivatives. The catalyst can be recovered and reused at least five times without loss of activity. The application of an eco-friendly, noncorrosive, and reusable catalytic system with high isolated yield of the products makes this method advantageous.
Introduction
Due to increasing concern about tight legislation, development of environmentally benign chemical processes has been of central importance in synthetic organic chemistry. In this regard, attention has been focused on use of alternative reaction media that can circumvent the problems of toxicity associated with volatile organic solvents. Water is the most environmental-friendly and least expensive solvent and, therefore, reactions mediated by water should constitute the ideal green chemistry Citation1–3. Various reactions such as aldol reaction, Michael reaction Citation4–6, Diels-Alder reaction, deprotection of acetates, alkyl ethers and acetals, allylation reaction Citation7, glycosidation Citation8, nucleophilic substitution reaction Citation9, synthesis of heterocycles Citation10–12, azidolysis, and polymerization Citation13 have been reported in aqueous medium Citation14, Citation15. In many of these reactions water not only acts as a greener solvent but also accelerates reaction rates and enhances reaction selectivity Citation16–19.
Lewis acids have been used to promote various reactions in organic synthesis. However, most of these conventional Lewis acids suffer from one or other drawback of corrosiveness, moisture sensitivity, recovery and reuse, environmental hazards, and waste control after the reaction. Thus, it is important to replace these acid catalysts with environmental-friendly catalysts which are active under mild conditions and can be easily recovered and reused. Metal triflates are an attractive alternative because of their low toxicity, noncorrosive nature, safety, ease to handle, recovery, and separability. Metal triflates are efficient and mild green Lewis acid catalysts that function in a variety of reaction Citation20, Citation21. Unlike traditional Lewis acids they are required in catalytic amount and are stable in presence of water. Barium triflate has been used for the preparation of vanadyl triflate Citation22.
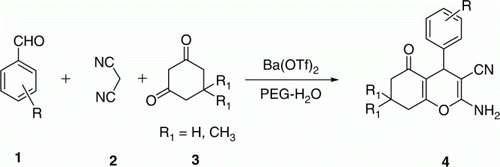
The 4H-chromene is an important structural motif and has received considerable importance in the field of medicinal chemistry due to wide range of useful biological activities. Substituted 4H-chromene derivatives are potent apoptosis inducing agents possessing vascular-disrupting activity Citation23, Citation24, they bind to Bcl-2 protein and induce apoptosis in tumor cells Citation25. These compounds have also been shown to possess anti-cancer and anti-coagulant activities Citation26 and important regulators for potassium cation channel Citation27. Due to their significant biological activities and their use in different fields there has been a great interest in developing synthetic protocols for these compounds. A widely used approach for the synthesis of 2-amino-chromenes is by refluxing malononitrile, aldehyde, and diketone in the presence of hazardous organic bases like piperidine, triethyl amine, pyridine etc. for several hours Citation28–31. In recent years new method have been developed for the synthesis of 4H-chromene-3-carbonitrile derivatives using various reagents such as d,l-proline Citation32, Citation33, MgO Citation34 Citation35, rare-earth perfluorooctanoate Citation36, ammonium salts Citation37–42, DBSA Citation43, LiBr Citation44, CeCl3·H2O Citation45, potassium phosphate Citation46, and different energy sources such as microwave-irradiation Citation47, Citation48 and ultrasonic irradiation Citation49, Citation50. Recently, a catalyst free method was reported for the synthesis of 4-pyrazolyl-4H-chromene derivatives in water Citation51. However, many of these methodologies are associated with several shortcomings such as expensive reagents, prolonged reaction times, high temperature, use of hazardous and chlorinated solvents, low product yield, huge organic waste, and difficulty in recovery and reuse of catalyst. In view of recent surge in use of greener solvent for organic transformations and develop an efficient and convenient method in terms of operational simplicity and economical viability, herein, we report a simple, efficient, and greener protocol for the synthesis of 4H-chromene-3-carbonitrile derivatives using Ba(OTf)2 as a catalyst in PEG-water ().
Results and discussion
In our preliminary attempts reaction of 4-chlorobenzaldehyde, malononitrile, and 5,5-dimethylcyclohexane-1,3-dione (dimedone) was selected as model reaction for the synthesis of 2-amino-5,6,7,8-tetrahydro-5-oxo-4-(4′-chlorophenyl)-4H-chromene-3-carbonitrile (4a). The model reaction was carried out in different solvents such as dimethylformamide (DMF), dimethylsulfoxide (DMSO), 1,2-dichloroethane, toluene, Tetrahydrofuran (THF), acetonitrile, ethanol, PEG-400, water, and different combination of PEG-water at room temperature using 20 mol% of Ba(OTf)2 as catalyst (). The desired product (4a) was only observed in DMF, DMSO, PEG-400, water, and PEG-water mixture whereas in 1,2-dichloroethane and toluene 4-chlorophenylidenemalononitrile intermediate was observed. In other organic solvents such as THF, acetonitrile, ethanol no product was formed under these conditions (, entry 5–7). Further, we studied model reaction in mixture of PEG-H2O in different ratio and found that the PEG:H2O (50:50 v/v) gave the highest yield (96%) of 4a at room temperature (, entry 10). In the absence of catalyst 4a was observed in 30 and 55% yield in water and PEG-water (50:50 v/v), respectively. The improved yield in PEG-H2O–Ba(OTf)2 system may be explained in terms of improved solubility of substrates and metal coordination ability of PEG. Addition of PEG as co-solvent in water is known to decrease apparently the aqueous solution polarity. It is assumed that PEG also acts as a phase transfer catalyst and helps in bringing the aqueous reagent and organic reagent together.
Table 1. Ba(OTf)2 catalyzed one-pot synthesis of 4H-chromene-3-carbonitrile (4a) in different solvents.a
Table 2. Synthesis of 2-amino-5,6,7,8-tetrahydro-5-oxo-4-(4-chlorophenyl)-4H-chromene-3-carbonitrile with different catalysts in PEG-water (4a).a
Next we screened the model reaction with different metal triflate (20 mol%) in PEG-water (50:50 v/v) and the yield of 4a is shown in . All of the metal triflates gave desired product in good to excellent yield, however, Ba(OTf)2 gave the highest yield (96%) among all screened catalysts. To optimize the catalysts loading we carried out model reaction with varying catalyst loading (). It was found that there was no appreciable increase in the yield of 4a by increasing the catalyst loading from 20 to 30 mol%, whereas by decreasing the catalyst loading from 20 to 10 mol% yield of 4a decreased from 96 to 80%. Further decrease in catalyst loading to 1 mol% lowered the yield of 4a to 55% (, entry 1). Thus, we took 20 mol% of Ba(OTf)2 as optimum amount for further studies.
Table 3. Effect of catalyst concentration on synthesis of 4H-chromene-3-carbonitrilea (4a).
To generalize the versatility of the catalyst, reaction of a variety of aldehydes and diketones with malononitrile were carried out under these conditions. It is noteworthy to mention that aldehydes with both electron withdrawing and donating groups reacted with different 1,3-diketones and malononitrile to give substituted 4H-chromene-3-carbonitrile in excellent yield ().
Table 4. Synthesis of substituted 4H-chromene derivatives (4a-r) catalyzed by Ba(OTf)2.a
It is expected that the reaction proceeds through the formation of arylidenemalononitrile by initial condensation of aromatic aldehyde with malononitrile. Formation of this intermediate was confirmed by comparing the TLC of Ba(OTf)2 catalyzed reaction between malononitrile and 4-chlorobenzaldehyde alone with that of reaction between malononitrile, dimedone and 4-chlorobenzaldehyde in PEG-water. Further, peak at 2358 and 2228 cm–1 in IR and peak at m/z 189.0037 in mass spectra of the intermediate isolated by quenching the reaction after 10 min confirmed the formation of 4-chlorophenylidenemalononitrile in model reaction. The Michael addition of the enolizable diketone to arylidenemalononitrile followed by intramolecular cyclization of the resulting species produced 2-amino-5,6,7,8-tetrahydro-5-oxo-4-aryl-4H-chromene-3-carbonitrile.
From an environmental point of view, it is desirable to minimize the amount of waste for each organic transformation. In this context, we recycled the catalyst for subsequent runs. To study the reusability, the recovered Ba(OTf)2 was reused for the synthesis of 4a. After extraction of 4a from PEG-water with ethyl acetate, the aqueous layer was charged with another lot of 1,3-diketone, aldehyde, and malononitrile and stirred the reaction for 30 min at room temperature. The reaction mixture was extracted by ethyl acetate and the combined organic layer was evaporated to give 4a in 93% yield. This cycle was repeated for five times. The catalyst showed good reactivity and yield even after five cycles with little deterioration in catalytic activity ().
Table 5. Recycling of catalyst Ba(OTf)2 for the synthesis of 4a.a
Experimental
General
The melting points of the products were determined by open capillaries on a Buchi apparatus and are uncorrected. The 1H and 13C NMR spectra were recorded on a Varian (500 MHz) spectrometer in DMSO-d 6 with 1H resonant frequency of 500 MHz and 13C resonant frequency of 125 MHz. The chemical shifts are expressed in ppm (δ) and coupling constants (J) in Hz. The Mass spectra were recorded on QSTAR® ELITE LX/MS/MS from applied biosystems. All the chemicals were purchased from Sigma-Aldrich, India and SD fine chemicals (India). The products were purified by column chromatography using silica gel (60–120 mesh). The homogeneity of the compounds was described by TLC on aluminium silica gel 60 F254 (Merck, Germany) detected by UV light (254 nm) and iodine vapors.
General experimental procedure for the synthesis of 2-amino-5,6,7,8-tetrahydro-5-oxo-4-phenyl-4H-chromene-3-carbonitrile
To a mixture of aldehyde (1.0 mmol), malononitrile (1.1 mmol), and dimedone (1.0 mmol) in PEG-H2O (2 mL) was added Ba(OTf)2 (0.2 mmol). The reaction mixture was stirred for appropriate time as given in . The reaction was monitored by TLC and on completion of the reaction the product was extracted with ethyl acetate (2×20 mL). The organic layer was dried with sodium sulfate and evaporated under reduced pressure. The crude product was recrystalized with ethanol or purified by column chromatography using silica gel and eluting with ethyl acetate and hexane. All the compounds were characterized by mass, 1H NMR, 13C NMR, spectroscopic data.
Spectral data of 4a–o
2-Amino-4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4a)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.37–7.29 (m, 2H), 7.19–7.12 (m, 2H), 7.03 (s, 2H), 4.18 (s, 1H), 2.49 (t, J=3.4 Hz, 2H), 2.23 (d, J=16.1 Hz, 1H), 2.09 (d, J=16.0 Hz, 1H), 1.02 (s, 3H), 0.93 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.1, 163.0, 158.9, 144.2, 131.6, 129.5, 128.7, 120.0, 112.8, 58.2, 50.4, 35.5, 32.2, 28.8, 27.3; ESI-MS (m/z): C18H17ClN2O2 328.0979, Found 329.0937 [M + H]+ and 331.0887 [M + 2 +H]+.
2-Amino-4-p-tolyl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4b)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.06 (d, J=8.1 Hz, 2H), 7.00 (d, J=8.0 Hz, 2H), 6.94 (s, 2H), 4.11 (s, 1H), 2.48 (m, 2H), 2.25–2.15 (m, 4H), 2.07 (d, J=16.1 Hz, 1H), 1.01 (s, 3H), 0.93 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.0, 162.7, 158.9, 142.2, 136.0, 129.3, 127.5, 120.2, 113.3, 58.9, 50.4, 35.6, 32.2, 28.9, 27.2, 21.0; ESI-MS (m/z): C19H20N2O2 308.1525, Found 309.1422 [M + H]+.
2-Amino-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4c)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 6.66 (d, J=8.2 Hz, 2H), 6.55 (s, 2H), 6.45 (d, J=8.2 Hz, 2H), 3.73 (s, 1H), 3.32 (s, 3H), 2.10 (m, 2H), 1.85 (d, J=16.1 Hz, 1H), 1.70 (d, J=16.0 Hz, 1H), 0.64 (s, 3H), 0.55 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.1, 162.6, 158.3, 142.2, 136.0, 128.6, 127.5, 120.2, 114.1, 58.9, 55.4, 50.4, 35.2, 32.2, 28.8, 27.2; ESI-MS (m/z): C19H20N2O3 324.1474, Found 324.1372 [M + H]+.
2-Amino-4-(3-chlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4d)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.31 (t, J=7.8 Hz, 1H), 7.24 (ddd, J=8.0, 2.1, 1.1 Hz, 1H), 7.14 (t, J=1.9 Hz, 1H), 7.12–7.07 (m, 1H), 7.06 (s, 2H), 4.19 (s, 1H), 2.51 (s, 2H), 2.23 (d, J=16.1 Hz, 1H), 2.11 (d, J=16.0 Hz, 1H), 1.01 (s, 3H), 0.94 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.1, 163.3, 159.0, 147.7, 133.3, 130.7, 127.5, 127.1, 126.4, 119.9, 112.5, 58.1, 50.4, 35.8, 32.3, 28.7, 27.3; ESI-MS (m/z): C18H17ClN2O2 328.0979, Found 329.0846 [M + H]+ and 331.0756 [M + 2+H]+.
2-Amino-4-(3-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4e)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.16 (t, J=7.9 Hz, 1H), 6.94 (s, 2H), 6.71 (d, J=8.1 Hz, 1H), 6.66 (d, J=7.6 Hz, 1H), 6.60 (s, 1H), 4.09 (s, 1H), 3.64 (s, 3H), 2.55–2.40 (m, 2H), 2.21 (d, J=16.0 Hz, 1H), 2.07 (d, J=16.1 Hz, 1H), 0.99 (s, 3H), 0.92 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.3, 164.9, 159.6, 158.9, 146.8, 129.9, 128.3, 120.2, 119.7, 114.1, 113.3, 111.8, 58.5, 55.3, 35.9, 32.2, 27.3, 26.3; ESI-MS (m/z): C19H20N2O3 324.1474, Found 324.1474 [M + H]+.
2-Amino-4-(4-nitrophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4f)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 8.17–8.08 (m, 2H), 7.52–7.28 (m, 2H), 7.15 (s, 2H), 4.35 (s, 1H), 2.52 (d, J=2.7 Hz, 2H), 2.24 (d, J=16.1 Hz, 1H), 2.08 (d, J=15.2, Hz, 1H), 1.02 (s, 3H), 0.94 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.1, 163.5, 159.0, 152.7, 146.7, 129.1, 124.1, 119.7, 112.2, 57.4, 50.3, 36.1, 32.3, 28.7, 27.4; ESI-MS (m/z): C18H17N3O4 339.1219, Found 340.1168 [M + H]+.
2-Amino-4-(3-nitrophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4g)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 8.09–8.03 (m, 1H), 7.96 (s, 1H), 7.64 (dt, J=10.3, 5.2 Hz, 1H), 7.59 (dd, J=12.1, 4.4 Hz, 1H), 7.16 (s, 2H), 4.40 (s, 1H), 2.54–2.48 (m, 2H), 2.25 (d, J=16.1 Hz, 1H), 2.08 (d, J=17.8, Hz, 1H), 1.00 (s, 3H), 0.95 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.2, 163.6, 159.1, 148.2, 147.4, 134.6, 130.4, 129.0, 122.2, 119.8, 112.2, 57.6, 50.3, 35.8, 32.3, 28.8, 27.2; ESI-MS (m/z): C18H17N3O4 339.1219, Found 340.1183 [M + H]+.
2-Amino-4-(pyridin-4-yl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4h)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 8.46 (s, 2H), 7.17–7.09 (m, 4H), 4.19 (s, 1H), 2.51 (s, 2H), 2.24 (d, J=16.1 Hz, 1H), 2.11 (d, J=16.0 Hz, 1H), 1.02 (s, 3H), 0.95 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.1, 163.7, 159.1, 153.4, 150.1, 123.0, 119.8, 111.9, 57.2, 50.3, 35.6, 32.3, 28.7, 27.4; ESI-MS (m/z): C17H17N3O2 295.1321, Found 295.1279 [M + H]+.
2-Amino-4-phenyl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4i)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.26 (t, J=7.2 Hz, 2H), 7.19–7.09 (m, 3H), 6.97 (s, 2H), 4.16 (s, 1H), 2.49 (dd, J=9.3, 2.8 Hz, 2H), 2.23 (d, J=16.1 Hz, 1H), 2.08 (d, J=16.1 Hz, 1H), 1.02 (s, 3H), 0.94 (s, 3H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.1, 162.9, 158.9, 145.2, 128.8, 127.6, 127.0, 120.1, 113.2, 58.8, 50.4, 36.0, 32.2, 28.8, 27.2; ESI-MS (m/z): C18H18N2O2 294.1368, Found 295.1279 [M + H]+.
2-Amino-4-(4-chlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4j)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.32 (d, J=8.2 Hz, 2H), 7.17 (d, J=8.3 Hz, 2H), 7.03 (s, 2H), 4.19 (s, 1H), 2.62–2.56 (m, 2H), 2.33–2.19 (m, 2H), 1.94 (dt, J=11.0, 5.5 Hz, 1H), 1.86 (dt, J=13.1, 7.8 Hz, 1H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.9, 165.0, 158.9, 144.2, 131.5, 129.5, 128.7, 120.0, 113.8, 58.1, 36.7, 35.4, 26.9, 20.2; ESI-MS (m/z): Calcd for C16H13ClN2O2 300.0666, Found 301.0687 [M + H]+ and 303.0657 [M + 2+H]+.
2-Amino-4-(3-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4k)
Colorless solid, 1H NMR (500 MHz, DMSO-d6) δ ppm: 8.06 (ddd, J = 8.1, 2.3, 1.1 Hz, 1H), 7.97 (t, J = 2.0 Hz, 1H), 7.69–7.63 (m, 1H), 7.59 (t, J = 7.9 Hz, 1H), 7.16 (s, 2H), 4.40 (s, 1H), 2.72–2.54 (m, 2H), 2.36–2.19 (m, 2H), 1.95 (dt, J = 16.7, 5.7 Hz, 1H), 1.91–1.83 (m, 1H); 13C NMR (125 MHz, DMSO-d6) δ ppm: 196.4, 165.5, 159.1, 159.0, 148.2, 147.5, 134.7, 130.4, 122.2, 119.8, 113.3, 57.5, 36.7, 35.8, 27.0, 20.2; ESI-MS (m/z): Calcd for C16H13N3O4 311.0906, Found 312.0768 [M + H]+.
2-Amino-4-p-tolyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4l)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ ppm: 7.06 (d, J=6.9 Hz, 2H), 7.04–6.99 (m, 2H), 6.94 (s, 2H), 4.13 (s, 1H), 2.58 (dd, J=11.7, 6.3 Hz, 2H), 2.23–2.26 (m, 2H), 2.23 (s, 3H), 1.93 (dd, J=11.5, 5.7 Hz, 1H), 1.90–1.79 (m, 1H); 13C NMR (125 MHz, DMSO-d 6 ) δ ppm: 196.2, 164.66, 158.8, 142.3, 136.0, 129.3, 127.5, 120.0, 114.4, 58.8, 36.9, 35.5, 26.9, 21.0, 20.3; ESI-MS (m/z): Calcd for C17H16N2O2 280.1212, Found 281.1247 [M + H]+.
2-Amino-4-(3-methoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4m)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.18 (td, J=8.2, 1.5 Hz, 1H), 6.97 (s, 2H), 6.83–6.59 (m, 3H), 4.14 (s, 1H), 3.71 (s, 3H), 2.70–2.51 (m, 2H), 2.34–2.17 (m, 2H), 2.04–1.77 (m, 2H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.3, 165.0, 159.6, 158.9, 146.8, 129.9, 120.2, 119.7, 114.1, 113.7, 111.8, 58.5, 55.3, 36.8, 35.7, 26.9, 20.3; ESI-MS (m/z): C17H16N2O3 296.1161, Found 297.1084 [M + H]+.
2-Amino-4-(4-methoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4n)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.04 (d, J=8.3 Hz, 2H), 6.92 (s, 2H), 6.81 (d, J=8.3 Hz, 2H), 4.11 (s, 1H), 3.69 (s, 3H), 2.62–2.51 (m, 2H), 2.36–2.13 (m, 2H), 2.01–1.74 (m, 2H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.3, 164.5, 158.3, 142.3, 136.1, 128.6, 127.4, 120.1, 114.1, 58.9, 55.4, 35.1, 35.5, 26.9, 20.3; ESI-MS (m/z): C17H16N2O3 296.1161, Found 297.1069 [M + H]+.
2-Amino-4-(3-chlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4o)
Colorless solid, 1H NMR (500 MHz, DMSO-d 6 ) δ 7.27 (t, J=7.6 Hz, 1H), 7.21 (ddd, J=8.1, 2.2, 1.2 Hz, 1H), 7.11 (t, J=1.85 Hz, 1H), 7.08–7.05 (m, 1H), 7.01 (s, 2H), 4.09 (s, 1H), 2.69–2.56 (m, 2H), 2.37–2.11 (m, 2H), 2.03–1.79 (m, 2H); 13C NMR (125 MHz, DMSO-d 6 ) δ 196.1, 163.3, 159.1, 143.1, 135.1, 131.0, 127.5, 127.2, 126.4, 120.0, 112.6, 58.0, 35.9, 32.3, 28.7, 20.2; ESI-MS (m/z): Calcd for C16H13ClN2O2 300.0666, Found 301.0685 [M + H]+ and 303.0675 [M + 2+ H]+.
Conclusions
In conclusion, we have developed an expeditious and greener synthesis of 4H-chromenes-3-carbonitrile derivatives by three-component one-pot condensation of 1,3-diketone, aldehyde and malononitrile using 20 mol% of Ba(OTf)2 as catalyst in PEG-water (50:50 v/v) at room temperature. Results from various reaction media and different metal triflates as catalysts show that choice of proper catalyst and the solvent system play a key role in the synthesis of 4H-chromene-3-carbonitrile derivatives. The catalyst can be recovered and reused at least five times without loss of activity. The application of an eco-friendly, non-corrosive, and reusable catalytic system with high isolated yield of the products makes this method advantageous.
Acknowledgements
This work was supported by Council of Scientific and Industrial Research (CSIR), New Delhi (01(2214)/08/EMR-II) and University Grant Commission (UGC), New Delhi under UGC-SAP programme.
References
- Sartori , G .; Ballini , R .; Bigi , F .; Bosica , G .; Maggi , R .; Righi , P . Chem. Rev . 2004 , 104 , 199 .
- Breslow , R . Acc. Chem. Res . 1991 , 24 , 159 .
- Rideout , D .; Breslow , R . J. Am. Chem. Soc . 1980 , 102 , 7816 .
- Shirakawa , S .; Kobayashi , S . Chem. Lett . 2006 , 1410 .
- Ding , R .; Katebzadeh , K .; Roman , L .; Bergquist , K.-E .; Lindström , U.M . J. Org. Chem . 2006 , 71 , 352 .
- Sharma , G .; Kumar , R .; Chakraborti , A.K . Tetrahedron Lett . 2008 , 49 , 4269 .
- Zha , Z .; Hui , A .; Zhou , Y .; Miao , Q .; Wang , Z .; Zhang , H . Org. Lett . 2005 , 7 , 1903 .
- Aoyama , S .; Kobayashi , S . Chem. Lett . 2006 , 238 .
- Shirakawa , S .; Kobayashi , S . Org. Lett . 2006 , 8 , 4939 .
- Selvam , N.P .; Saravanan , C .; Muralidharan , D .; Perumal , P.T . J. Heterocylic. Chem . 2006 , 43 , 1379 .
- Shanthi , G .; Perumal , P.T . Synlett , 2008 , 2791 .
- Shelke , K.F .; Sapkal , S.B .; Kakade , G.K .; Sadaphal , S.A .; Shingate , B.B ; Shingare , M.S . Green Chem. Lett. Rev . 2010 , 3 , 17 .
- Satoh , K .; Kamigaito , M .; Sawamoto , M . Macromolecules 2000 , 33 , 5836 .
- Hailes , H.C . Org. Proc. Res. Dev . 2007 , 11 , 114 .
- Li , C.J .; Chen , L . Chem. Soc. Rev . 2006 , 35 , 68 .
- Khatik , G.L .; Kumar , R .; Chakraborti , A.K . Org. Lett . 2006 , 8 , 2433 .
- Grieco , P.A .; Brandes , E.B .; McCann , S .; Clark , J.D . J. Org. Chem . 1989 , 54 , 5849 .
- Chakraborti , A.K .; Rudrawar , S .; Jadhav , K.B .; Kaur , G .; Chankeshwara , S.V . Green Chem . 2007 , 9 , 1335 .
- Chankeshwara , S.V .; Chakraborti , A.K . Org. Lett . 2006 , 8 , 3259 .
- Kobayashi , S .; Manabe , K . Pure Appl. Chem . 2000 , 72 , 1373 .
- Surya Prakash , G.K .; Mathew , T .; Panja , C .; Alconcel , S .; Vaghoo , H .; Do , C .; Olah , G.A . Proc. Natl. Acad. Sci. USA 2007 , 104 , 3703 .
- De , S.K . J. Mol. Catal. A: Chem . 2005 , 226 , 77 .
- Kemnitzer , W .; Kasibhatla , S .; Jiang , S .; Zhang , H .; Wang , Y .; Zhao , J .; Jia , S .; Herich , J .; Labreque , D .; Storer , R .; Meerovitch , K .; Bouffard , D .; Rej , R .; Denis , R .; Blais , C .; Lamothe , S .; Attardo , G .; Gourdeau , H .; Tseng , B .; Drewe , J .; Cai , S.X . J. Med. Chem . 2004 , 47 , 6299 .
- Kasibhatla , S .; Gourdeau , H .; Meerovitch , K .; Drewe , J .; Reddy , S .; Qiu , L .; Zhang , H .; Bergeron , F .; Bouffard , D .; Yang , Q .; Herich , J .; Lamothe , S .; Cai , S.X .; Tseng , B . Mol. Cancer Ther . 2004 , 3 , 1365 .
- Wang , J .; Liu , D .; Zhang , Z .; Shan , S .; Han , X .; Srinivasula , S .; Croce , C .; Alnemri , E .; Huang , Z . Proc. Natl. Acad. Sci. USA 2000 , 97 , 7124 .
- Bonsignore , L .; Loy , G .; Secci , D .; Calignano , A . Eur. J. Med. Chem . 1993 , 28 , 517 .
- Jin , T.S .; Wang , A.Q .; Wang , X .; Zhang , J.S .; Li ., T.S . Synlett 2004 , 871 .
- Harb , A.F .; Hesien , A.M .; Metwally , S.A .; Elnagdi , M.H . Liebigs Ann. Chem . 1989 , 585 .
- Martin , N .; Pascual , C .; Seoane , C .; Soto , J.L . Heterocycles 1987 , 26 , 2811 .
- Elagamay , A.G.A .; El-Taweel , F.M.A.A . Indian J. Chem . 1990 , 29B , 885 .
- Zayed , S.E .; AbouEImaged , E.I .; Metwally , S.A .; Elnagdi , M.H.H . Collect. Czech. Chem. Commun . 1991 , 56 , 3093 .
- Guo , S.-B .; Wang , S.-X .; Li , J.-T . Synth. Commun . 2007 , 37 , 2111 .
- Balalaie , S .; Bararjanian , M .; Amani , A.M .; Movassagh , B . Synlett 2006 , 263 .
- Mohammad , S .; Hassan , S . Catalysis Lett . 2008 , 126 , 275 .
- Kumar , D .; Reddy , V.B .; Sharad , S .; Dube , U .; Kapur , S . Eur. J. Med. Chem . 2009 , 44 , 3805 .
- Wang , L.M .; Shao , J.H .; Tian , H .; Wang , Y.H .; Liu , B . J. Fluorine Chem . 2006 , 127 , 97 .
- Ranu , B.C .; Banerjee , S .; Roy , S . Indian J. Chem . 2008 , 47B , 1108 .
- Shi , D .; Mou , J .; Zhuang , Q .; Wang , X . J. Chem. Res . 2004 , 821 .
- Gao , S .; Tsai , C.H .; Tseng , C .; Yao , C.F . Tetrahedron 2008 , 64 , 9143 .
- Chen , L .; Li , Y.Q .; Huang , X.J .; Zheng , W.J . Heteroatom Chem . 2009 , 20 , 91
- Balalaie , S .; Sheikh-Ahmadi , M .; Bararjanian , M . Catal. Commun . 2007 , 8 , 1724 .
- Jin , T.S .; Wang , A.Q .; Wang , X .; Zhang , J.S .; Li , T.S . Synlett 2004 , 871 .
- Jin , T.S .; Wang , A.Q .; Zhang , J.S .; Zhang , F.S .; Li , T.S . Chinese J. Org. Chem . 2004 , 24 , 1598 .
- Sun , W.B .; Zhang , P .; Fan , J .; Chen , S.H .; Zhang , Z.H . Synth. Commun . 2010 , 40 , 587 .
- Sabitha , G .; Arundhathi , K .; Sudhakar , K .; Sastry , B.S .; Yadav J.S . Synth. Commun . 2009 , 39 , 433 .
- Pore , D.M .; Undale , K.A .; Dongare , B.B .; Desai , U.V . Catalysis Lett . 2009 , 132 , 104 .
- El-Rahaman , N.M.A .; El-Kateb , A.A .; Mady , M.F . Synth. Commun . 2007 , 37 , 3961 .
- Tu , S.J .; Gao , Y .; Guo , C .; Shi , D .; Lu , Z . Synth. Commun . 2002 , 32 , 2137 .
- Li , J.T .; Xu , W.Z .; Yang , L.C .; Li , T.S . Synth. Commun . 2004 , 34 , 4565 .
- Tu , S.J .; Jiang , H .; Zhuang , Q.Y .; Miao , C.B .; Shi , D.Q .; Wang , X.S .; Gao , Y.M . Chinese J. Org. Chem . 2003 , 23 , 488 .
- Kumaravel , K .; Vasuki , G . Green. Chem . 2009 , 11 , 1945 .
- Zhi , H .; Lue , C .; Zhang , Q .; Luo , J . Chem. Commun . 2009 , 2878 .