Abstract
Anilines react with two equivalents of allyl bromide in aqueous ethanol in the presence of Mg–Al hydrotalcites (Mg/Al = 5, 3, 2) at room temperature to give N,N-diallylanilines in good yield. Hydrotalcites (HTs) are mild and efficient catalysts, which can be easily recovered and reused. In this project, efforts are made to check the activity of the calcined and uncalcined HTs. Also, the methodology can be extended for the synthesis of various N,N-diallylanilines.
Introduction
N,N-diallylanilines are an important class of compounds which are precursors of nitrogen heterocycles, such as lactams, pyrroles, indoles, and quinolines. These compounds possess a number of biological activities and have been found to show antimalarial, antibacterial, antiasthmatic, antihypertensive, and antiinflammatory activities Citation1. They are also precursors to many natural products Citation2. Olefin metathesis has given an impetus to the preparation of the bisallylated compounds as they are useful precursors for the ring closing metathesis to give N-aryldihydropyrroles Citation3.
N-allylation of aniline is carried out by the reaction of aniline and allyl bromide in the presence of an acid scavenger, for example Na2CO3 in ethanol Citation4, sodium lauryl sulfate, NaHCO3 in water Citation5, PhLi in diethyl etherCitation6, N(Et)3 in acetonitrile Citation7, Al2O3 zeolite KY in benzeneCitation8, and KOH in THFCitation9. It is also carried out by the reaction of aniline and allyl alcohol using platinum complexes, e.g. Pt(eta3-allyl)(xantphos)]OTf in DMF Citation10. Pt(COD)Cl2, bis[2-(diphenylphosphino)phenyl ether in dioxane Citation11 or HgO–HBF4 in THF Citation12. There are few reports on the N-allylation reaction using palladium complex catalyst, for example 1,2-(p-MeOC6H4)2cyclobuten[=P(2,4,6-tri-t-BuPh)]2 PdOTf in toluene Citation13, Mo3PdS4 Citation14, Pd·Et3B Citation15, Ti(O-iPr)4 Pd(OCOCF3)2-dppb in benzene Citation16, and Pd{P(OC6H5)3}4 in toluene Citation17. There is also a recent report on N-allylation of amines using silica as a heterogeneous catalyst Citation18. Though, better results are obtained using homogeneous liquid phase catalysts; these are not environmentally benign. Some of the aforementioned procedures use harsh reaction conditions, use expensive catalysts, and require longer reaction time. Also, catalysts recovery and recyclability is the major issue encountered. Further, the methods are not atom economic. Considering the importance of diallylanilines, a green and efficient method is needed.
Hydrotalcites (HTs) are anionic clays having basic properties. Mg/Al HTs are commonly used as solid base catalysts for a number of organic reactions Citation19 Citation20. Herein we report a greener method for the preparation of N,N-diallylanilines by the reaction of aniline with allyl bromide in the presence of a Mg/Al HTs as solid bases in aqueous ethanol at ambient temperature.
Results and discussion
The reaction of aniline and two equivalents of allyl bromide was carried out without catalyst in aq. ethanol (50% v/v). It was found that the conversion of the aniline was low even after 90 minutes and a mixture of monoallyl and diallyl anilines was formed (Entry 1, ). Mg/Al HTs with different Mg/Al ratios, HT5, HT3, and HT2, were prepared corresponding to Mg/Al=5, 3, 2 by the reported procedure Citation21. The HTs were calcined at 550°C to obtain porous metal oxides (HT5C, HT3C, and HT2C). To study the effect of calcined and uncalcined catalyst, the reaction of aniline and two equivalents of allyl bromide was carried in aq. ethanol (50% v/v) (). All the HTs were found to catalyze the reaction and among them HT5 was found to be the best catalyst which could be reused. There is no much difference in the yield of the product catalyzed by calcined and uncalcined catalyst. The possible reason may be the reaction requires minimum basicity. Also, there is difference in the yields obtained in substrate study (); the reason here could be the catalysts may be acting as a scavenger of the acid generated in the reaction. In these reactions, diallylanilines were obtained as the sole product; monoallylaniline was found in traces.
Table 1. Reaction of aniline and allyl bromide in the presence of different HTs.
The reaction was carried out using different quantities of HT5 (). The catalyst (20 wt.%) with respect to aniline was found to be optimum, where the aniline conversion was maximum. There was no further increase in the product yield, after increasing the catalyst loading.
Table 2. Reaction of aniline and allyl bromide in the presence of different quantities of HT5.
To check the reusability, recovered HT5 was reused in the reaction of aniline and allyl bromide (). After each cycle, the catalyst was filtered, washed with aq. NaHCO3, ethyl acetate, and water. After washing, the catalyst was air dried at 60°C and used for the successive cycle. It was found that the catalyst (HT5) could be recycled up to three runs.
Table 3. The reaction of aniline and allyl bromide in the presence of HT5.
Using the optimized quantity of HT5, different anilines were reacted with two equivalent of allyl bromide in ethanol at room temperature to obtain the corresponding N,N-diallyl anilines (). The reaction of p-nitroaniline at room temperature was sluggish (Entry 4), so the reaction was carried out at 80°C. In this reaction, the diallyl derivative (44%) was formed, along with the monoallyl derivative. The reaction of ortho-trifluromethyl aniline with allyl bromide also needed high temperature and was carried out at 80°C. In this reaction, only 10% of the diallyl derivative was formed, while 70% of the monoallyl derivative was formed. The products were isolated by column chromatography and characterized by GC–MS and 1H NMR.
Table 4. Reaction of anilines and allyl bromide in the presence of HT5.
Experimental
1H NMR spectra of the compounds were recorded on Bruker 400 MHz. GC–MS analysis on Shimadzu-QP 2010 GC–MS having Rtx wax capillary column, 30-m length, 0.32-mm diameter, and 0.24-mm inner diameter.
Catalyst preparation
Mg–Al HT-2
An aqueous solution (100 mL) containing Mg(NO3)2·6H2O (25.64 g, 100 mmol) and Al(NO3)3·9H2O (18.76 g, 50 mmol) was added drop wise using dropping funnel to a vigorously stirred solution aqueous solution (100 mL) containing NaOH (12 g, 300 mmol) and Na2CO3 (10.06 g, 100 mmol) taken in a two-necked round bottom flask. After complete addition, the solution was heated at 80°C for 18 h. The solution was allowed to cool to room temperature and filtered. The residue was washed with distilled water several times till the filtrate was neutral (pH paper). The solid was dried in an oven at 60°C in air (electric oven).
Calcination of the dried catalyst at 550°C gave porous metal oxides (HT5C).
Catalysts Mg–Al HT-3 and Mg–Al HT-5 were prepared using above procedure Citation21.
General procedure for diallylation of anilines
Aniline (10 mmol), allyl bromide (20 mmol), and HT5 (20 wt.% of aniline) in aqueous ethanol (50% v/v, 20 mL) were stirred in a 25 mL round bottom flask at room temperature. After completion of the reaction, ethanol was evaporated. Ethyl acetate (5 mL) was added to the reaction mass and catalyst was filtered through G4 sintered glass funnel. The solid catalyst was washed with ethyl acetate (2 mL). The filtrate obtained was treated with aq. NaHCO3 (5 mL, 20%).
Part A (Tables ): The extract was dried over Na2SO4 and was collected in a 10 mL-standard volumetric flask. On final dilution, the sample was taken for the GC analysis. The conversion of the reactant and the yield were calculated based on the authentic compounds. GC Analysis was done on Chemito Gas Chromatogram.
Model: GC1000; Column specification: BP10 capillary column, 30-m length, 0.32-mm diameter, and 0.24-µm inner diameter. Detector temperature: 250°C, oven temperature 240°C, Programme: 80°C-0 min-5°C/min-240°C, Flame type: FID.
Part B (): The extract was evaporated after drying over Na2SO4. The product was purified by column chromatography on silica gel and pet ether as an eluent. The products 1Citation22, 2Citation22, 3Citation18, 4Citation18, 5Citation18, 6Citation2, 7Citation8 and 10Citation23 are reported. The products 8 and 9 are new and are characterized by 1H NMR and GC–MS, respectively.
Spectral data
1a: N,N -diallylaniline
Mass spectrum: (C12H15N) 173 (M+) (base), 158, 146, 130, 117, 104, 91, 51, 41.
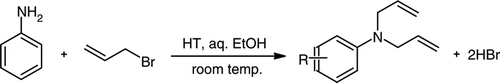
1 H NMR (CDCl3) d H: 3.89–4.00 (m, 4H, 2CH2), 5.17–5.23(m, 4H, 2CH2=), 5.84–5.94 (m, 2H, 2CH = ), 6.69–6.74 (m, 2H, 3ArH), 7.21–7.2 (m, 2H, 2ArH) ppm.
2a: N,N -diallyl-4-methylaniline
Mass spectrum: (C13H17N). 187 (M+) (base), 172, 160, 144, 130, 118, 105, 91, 77, 65.
3a: N,N -diallyl-4-methoxyaniline
Mass spectrum: (C13H17NO). 203 (M+) (base), 188, 176, 162, 146, 135, 120, 107, 92, 77, 65.
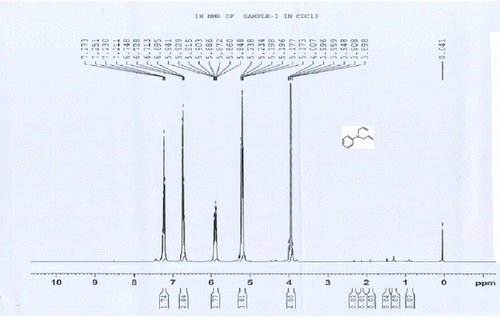
1 H NMR (CDCl3) d H: 3.75 (s, 3H, CH3), 3.81–3.92 (m, 4H, 2CH2), 5.14–5.21(m, 4H, 2CH2=), 5.82–5.90 (m, 2H, 2CH = ), 6.68–7.26 (m, 4H, 4ArH) ppm.
4a: N,N -diallyl-4-nitroaniline
Mass spectrum: (C12H14NO) 218 (M+) (base), 203, 191, 171, 157, 145, 130, 117, 103, 91, 77.
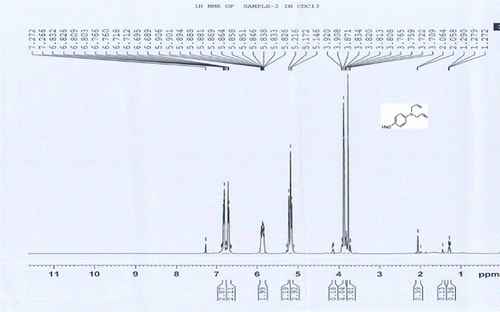
1 H NMR (CDCl3) d H: 3.97–4.07 (m, 4H, 2CH2), 5.15–5.30 (m, 4H, 2CH2), 5.80–5.89 (m, 2H, 2CH = ), 6.57–6.69 (m, 2H, 2ArH), and 8.09–8.16 (m, 2H, 2ArH) ppm.
5a: N,N -diallyl-4-bromoaniline
Mass spectrum: (C12H10BrN) 251 (1:1), M+) (base), 226, 224, 210, 186, 182, 157, 145, 130, 117, 103, 90, 76, 63, 50.
1 H NMR (CDCl3) d H: 3.85–3.96 (m, 4H, 2CH2), 5.14–5.24 (m, 4H, 2CH2=), 5.79–5.88 (m, 2H, 2CH = ), 6.53–6.63 (m, 2H, 2ArH), and 7.27–7.32 (m, 2H, 2ArH) ppm.
6a: N,N -diallyl-4-chloroaniline
Mass spectrum: (C12H14ClN). 207 (M+) (base), 182, 180, 164, 140, 130, 113, 111, 89, 75, 63, 51.
7a: N,N -diallyl-4-fluroaniline
Mass spectrum: (C12H14FN). 191 (M+) (base), 176, 164, 148, 135, 122, 109, 95, 75, 68.
8a: 1-(4-(diallylamino)phenyl)ethanone
Mass spectrum: (C14H17NO) 215 (M+) (base), 200, 188, 172, 158, 146, 130, 118, 103, 91,77, 65.
1 H NMR (CDCl3) d H: 2.5 (s, 3H, 1CH3), 3.94–4.05 (m, 4H, 2CH2), 5.14–5.26 (m, 4H, 2CH2=), 5.81–5.90 (m, 2H, 2CH = ), 6.60–6.72 (m, 2H, 2ArH), and 7.80–7.99 (m, 2H, 2ArH) ppm.
9a: N,N -diallyl-2-(trifluoromethyl)aniline
Mass spectrum: (C13H14F3N). 201 (M+) (base), 180, 154, 145, 127, 114, 95, 77, 65.
10a: 4-(diallylamino)phenol
Mass spectrum: (C12H15NO). 189 (M+) (base), 174, 162, 146, 133, 120, 1075, 93, 77, 65.
Conclusion
Aromatic primary amines can be effectively diallylated by reacting them with two equivalents of allyl bromide in the presence of Mg/Al HTs (Mg/Al= 5) at room temperature in aqueous ethanol. This method is environmentally benign, convenient to carry out, and the catalyst is recyclable up to three cycles.
 1a:
1
H NMR of N,N-bisallylaniline
1a:
1
H NMR of N,N-bisallylaniline
1 HNMR (CDCl3) δH: 3.89–4.00 (m, 4H, 2CH2), 5.17–5.23 (m, 4H, 2CH2 = ), 5.84–5.94 (m, 2H, 2CH = ),6.69–6.74 (m, 2H, 3ArH = ), 7.21–7.2 (m, 2H, 2ArH) ppm.
3a: 1H NMR of 4-methoxy N,N-bisallylaniline
1 H NMR (CDCl3) δH: 3.75 (s, 3H, CH3), 3.81–3.92 (m, 4H, 2CH2), 5.14–5.21 (m, 4H, 2CH2 = ), 5.82–5.90 (m, 2H, 2CH = ), 6.68–7.26 (m, 4H, 4ArH) ppm
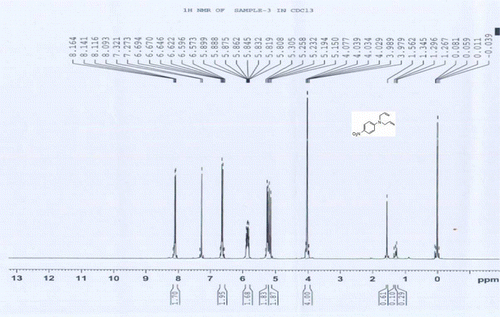
4a: 1H NMR of 4-nitro N,N-bisallylaniline
1H NMR (CDCl3) δH: 3.97–4.07 (m, 4H, 2CH2), 5.15–5.30 (m, 4H, 2CH2), 5.80–5.89 (m, 2H, 2CH = ), 6.57–6.69 (m, 2H, 2ArH), 8.09–8.16 (m, 2H, 2ArH) ppm.
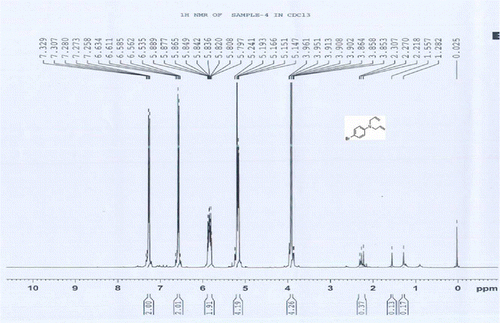 5a:
1H NMR of 4-bromo N,N-bisallylaniline
5a:
1H NMR of 4-bromo N,N-bisallylaniline
1H NMR (CDCl3) δH: 3.85–3.96 (m, 4H, 2CH2), 5.14–5.24 (m, 4H, 2CH2 = ), 5.79–5.88 (m, 2H, 2CH = ), 6.53–6.63 (m, 2H, 2ArH), 7.27–7.32 (m, 2H, 2ArH) ppm.
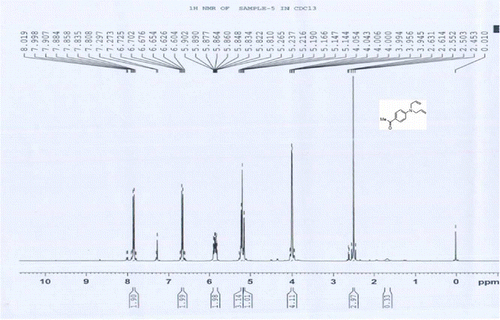
8a 1H NMR of 1-(4-(diallylamino)phenyl)ethanone
1H NMR (CDCl3) δH: 2.5 (s, 3H, 1CH3), 3.94–4.05 (m, 4H, 2CH2), 5.14–5.26 (m, 4H, 2CH2 = ), 5.81–5.90 (m, 2H, 2CH = ), 6.60–6.72 (m, 2H, 2ArH), 7.80–7.99 (m, 2H, 2ArH) ppm.
Acknowledgements
MRS is thankful to the UGC, New Delhi, for a fellowship under UGC-SAP programme.
References
- Li , L. and Jones , W.D. 2007 . Mechanistic Investigation of the Cobalt-Catalyzed Selective Conversion of Diallylanilines to Quinoline Involving C–N and C–H Activations . J. Am. Chem. Soc. , 129 : 10707 – 10713 .
- Yang , S. and Chung , W. 1999 . Palladium Catalyzed N-Allylation of Anilines by Direct Use of Allyl Alcohols in the Presence of Titanium (IV) Isopropoxide . Tetrahedron Lett. , 40 : 953 – 956 .
- Adak , L. , Chattopadhyay , K. and Ranu , B.C. 2009 . Palladium Nanoparticle-Catalyzed C-N bond Formation. A Highly Regio- and Streoselective Allylic Amination by Allyl Acetates . J. Org. Chem. , 74 : 3983 – 3985 .
- Li , L. and Jones , W.D. 2007 . Mechanistic Investigation of the Cobalt-Catalyzed Selective Conversion of Diallylanilines to Quinoline Involving C–N and C–H activations . J. Am. Chem. Soc. , 129 : 10707 – 10713 .
- Singh , C.B. ; Kavala , V. ; Samal , A.K. ; Patel , B.K. Aqueous-Mediated N-Alkylation of Amines . Eur. J. Org. Chem . 2007 , 1369 – 1377 .
- Barluenga , J. , Najera , C. and Yus , M. A . 1980 . Convenient Synthesis of Substituted Piperazines via Aminomercuration-Demercuration of Diallylamines . J. Heterocycl. Chem. , 17 : 917 – 921 .
- Ghosh , D. , Thander , L. , Ghosh , S.K. and Chattopadhyay , S.K. 2008 . Sequential Aza-Claisen Rearrangement and Ring-Closing Metathesis as a Route to 1-Benzazepine Derivatives . Synlett. , 19 : 3011 – 3015 .
- Onaka , M. , Umezono , A. , Kawai , M. and Izumi , Y. 1985 . N-Alkylation of Aniline Derivatives by Use of Potassium Cation-Exchanged Y-Type Zeolite . J. Chem. Soc. Chem. Commun. , 17 : 1202 – 1203 .
- Martinez , V. , Blais , J.-C. and Astruc , D. 2002 . General Route from Simple Methyl, Alkyl, and Cycloalkyl Arenes to Polycyclic Cyclopentenyl Aryl Derivatives. The CpFe Group as an Activator and Tag . Org. Lett. , 4 : 651 – 654 .
- Ohshima , T. , Miyamoto , Y. , Ipposhi , J. , Nakahara , Y. , Utsunomiya , M. and Mashima , K. 2009 . Platinum-Catalyzed Direct Amination of Allylic Alcohols Under Mild Conditions: Ligand and Microwave Effects, Substrate Scope, and Mechanistic Study . J. Am. Chem. Soc. , 131 : 14317 – 14328 .
- Utsunomiya , M. , Miyamoto , Y. , Ipposhi , J. , Ohshima , T. and Mashima , K. 2007 . Direct Use of Allylic Alcohols for Platinum-Catalyzed Monoallylation of Amines . Org. Lett. , 9 : 3371 – 3374 .
- Barluenga , J. , Perez-Prieto , J. and Asensio , G. 1990 . Catalytic Aminomercuration of Olefins in a Tandem Aminomercuration-Deoxymercuration; One-Step Synthesis of Secondary n-Arylallylamines from Allylalcohols . Tetrahedron. , 46 : 2453 – 2460 .
- Ozawa , F. , Okamoto , H. , Kawagishi , S. , Yamamoto , S. , Minami , T. and Yoshifuji , M. 2002 . (π-Allyl)Palladium Complexes Bearing Diphosphinidene-Cyclobutene Ligands (DPCB): Highly Active Catalysts for Direct Conversion of Allylic Alcohols . J. Am. Chem. Soc. , 124 : 10968 – 10969 .
- Tao , Y. , Zhou , Y. , Qu , J. and Hidai , M. 2010 . Highly Ef?cient and Regioselective Allylic Amination of Allylic Alcohols Catalyzed by [Mo3PdS4] Cluster . Tetrahedron Lett. , 51 : 1982 – 1984 .
- Kimura , M. , Futamata , M. , Shibata , K. and Tamaru , Y. 2003 . Pd·Et3B-Catalyzed Alkylation of Amines with Allylic Alcohols . Chem. Commun. , 2 : 234 – 235 .
- Yang , S. and Chung , W. 1999 . Palladium Catalyzed N-Allylation of Anilines by Direct Use of Allyl Alcohols in the Presence of Titanium (IV) Isopropoxide . Tetrahedron Lett. , 40 : 953 – 956 .
- Kayaki , Y. , Koda , T. and Ikariya , T. 2004 . Halide-Free Dehydrative Allylation Using Allylic Alcohols Promoted by a Palladium Triphenyl Phosphite Catalyst . J. Org. Chem , 69 : 2595 – 2597 .
- Basu , B. , Paul , S. and Nanda , A.K. 2009 . Highly Selective N-Alkylation of Amines Promoted on Silica: An Ef?cient and Recyclable Surface . Green Chem. , 11 : 1115 – 1120 .
- Shen , J. , Tu , M. and Hu , C. 1998 . Structural and Surface Acid/Base Properties of Hydrotalcite-Derived MgAlO Oxides Calcined at Varying Temperatures . J. Solid State Chem. , 137 : 295
- Sels , F.B. , Vos , D.E. De. and Jacobs , P.A. 2001 . Hydrotalcite-Like Anionic Clays in Catalytic Organic Reactions . Catal. Rev. , 43 : 443
- Cavani , F. , Trifiro , F. and Vaccari , A. 1991 . Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications . Catal. Today. , 11 : 173
- Kang , K.H. , Choi , K. , Koh , H.Y. , Kim , Y. , Chung , B.Y. and Cho , Y.S. 2001 . Indium Mediated Allylation of Nitro Group on Nitrobenzene Derivatives in Aqueous Media . Synth. Commun. , 31 : 2277 – 2286 .
- Nakagawa , H. , Hirabayashi , T. , Sakaguchi , S. and Ishii , Y. 2004 . Allylation of Alcohols and Carboxylic Acids with Allyl Acetate Catalyzed by [Ir(cod)2] + BF4- Complex . J. Org. Chem. , 69 : 3474 – 3477 .