Abstract
Corrosion inhibition of aluminium in 1 M HCl by coconut coir dust extract (CCDE) was studied using weight loss and hydrogen evolution techniques at 30 and 60°C. It was found that the studied extract exhibits a very good performance as inhibitor for aluminium corrosion in 1 M HCl. Results show that the inhibition efficiency increases with increasing temperature and concentration of the extract. Inhibitive effect was afforded by adsorption of the extracts' components which was found to accord with Langmuir adsorption isotherm. Inhibition mechanism is deduced from the temperature dependence of the inhibition efficiency and was further corroborated by the values of activation parameters obtained from the experimental data.
1. Introduction
Aluminium is the most commonly used metal in power transmission and the metallurgy of nonferrous metals due to its high-electrical conductivity, good working and forming properties, low density, lightness, ease of recycling, high-mechanical strength, and ductility Citation1. Although aluminium has a protective oxide film, which contributes to its resistance to corrosion to a great variety of chemical agents Citation2, corrosion inhibitors should be used to improve its corrosion resistance because, according to the Pourbaix diagram, the solubility of the oxide film increases below pH 4.
The pickling, chemical, and the electrochemical etching of aluminium are carried out with hydrochloric acid solutions. The solubility of the protective oxide film upon the Al surface increases above and below pH 4–9 Citation3–5 range and aluminium exhibits a uniform attack. Inhibitors are used to prevent metal dissolution and minimize acid consumption. A number of organic compounds have been described as aluminium corrosion inhibitors in hydrochloric acid solution Citation6–11. Although most of the organic compounds have high inhibitive performance, their high cost and toxicity are the main drawbacks in the use of these compounds as corrosion inhibitors Citation12. In recent times most corrosion inhibitors studies have been focused on the development of “environmental friendly” compounds in response to legislation changes concerning environmental protection. This term includes formulations that are not toxic to humans, have low environmental impact, optimal biodegradability, and maintain their efficiency and cost-effectiveness. Thus, since the 1990s, many investigations have been related to the evaluation of natural compounds as corrosion inhibitors; in this sense, some amino acids, vitamins, plant extracts, and soluble natural polymers have been tested Citation13–22. In our laboratory, we have investigated and reported on the corrosion inhibition of aluminium in alkaline/acidic media using plant extracts and natural polymers Citation23–28.
In the extraction of coir fiber from the coconut husk and in the production of finished materials from the extracted fiber, a large amount of coir dust is produced. Coconut coir dust is described as that brown, spongy particle of low weight which falls out when the fiber is shredded from the husk. The coir dust is about 70% of the weight of the coconut husk Citation29. Coir dust is rich in lignins and tannins. It is reported to be composed of cellulose, pentosan, furfural, and lignin Citation30 Citation31. While a great deal has already been learned about the solid parts of the coconut, coir dust has, up to now, the least use and is still considered waste and nuisance for which no important industrial uses have been developed, and they are normally incinerated or dumped without control Citation32 Citation33. It is known to have no commercial importance except, may be, in applications where sawdust is used in a very limited amount. However, in an effort to find immediate solution to the perennial problem of coir dust disposal, several product development activities were undertaken that may bring about the large scale utilization of this waste material. For sometime, coir dust was used in the tropics as a locally available material for preparing soilless growing media for containerized crop production Citation34 Citation35. Only during the past few years has this material become commercially popular and it is now being successfully used in different parts of the world as an environmentally sound peat substitute for container-grown ornamental plants Citation36 Citation37.
Although there have been many research reports on the natural products as corrosion inhibitor for aluminium in aggressive solutions Citation38–42, but no published information to our knowledge is available on extract of coconut coir dust as corrosion inhibitor for any metal. The aim of this present work is to investigate the corrosion inhibiting effect of coconut coir dust extract (CCDE) at 30–60°C using weight loss and hydrogen evolution methods as part of our contribution to the growing interest on environmental friendly corrosion inhibitors as well as a means of practically exploiting this abundant natural resource.
2. Results and discussion
2.1. FTIR analysis of CCD extract
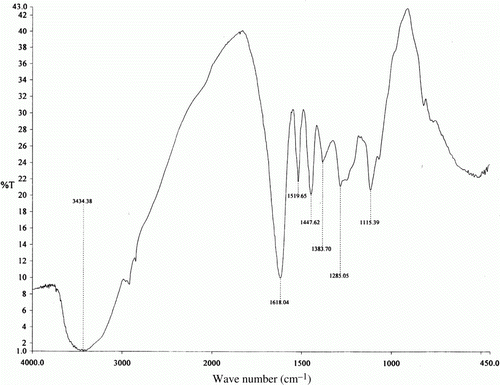
It is well established that FTIR spectrophotometer is a powerful tool that can be used to identify the type of bonding particularly functional group(s) present in organic compounds. shows the IR spectrum of the acetone extract of coconut coir dust. Original absorption at 3434.38 cm−1 (associated hydroxyl) was overlapped by the strong stretching mode of N–H. The 1618.06 cm−1 band is assigned to the N–H bend. The peak at 1285.05 cm−1 can be assigned to stretching mode of C–N group. The bands at 1447.62 cm−1 are attributed to C–C in ring (for aromatic). The absorption band at 1519.65 cm−1 is assigned to the N–O Asymmetric stretch and 1115.39 cm−1 is assigned to C–O stretch. This shows that this plant extracts contains mixtures of compounds, that is, alkaloids, flavonoids, organic acids, and so on Citation43.
2.2. Weight loss, corrosion rates, and inhibition efficiency
The electrochemistry of corroding metals involves two or more half-cell reactions. For aluminium in acid solutions, the corrosion reaction proceeds via two possible reactions namely, the partial anodic reaction that can be represented by the dissolution of metal according to the following:
and the partial cathodic reaction which can be represented by the overall reaction:
The overall chemical reaction is the sum of these two half-cell reactions and can be represented by:
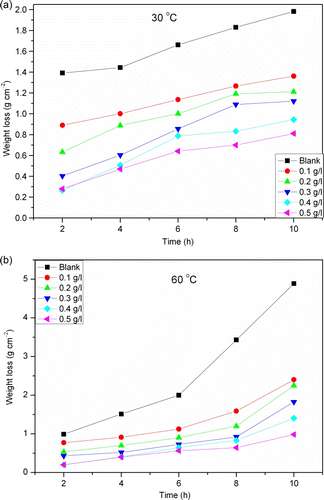
The overall chemical reaction in Equation (Equation3) suggests four ways to determine the corrosion rate by chemical (nonelectrochemical) means. These are (1) to determine the quantity of solid aluminium lost due to corrosion by measuring the weight loss (or thickness lost) of the metallic aluminium specimen, (2) to measure the concentration of dissolved Al3 + ions which are produced in solution, (3) to determine the quantity of hydrogen gas which is produced by the corrosion reaction, and (4) to determine pH changes in solution caused by the consumption of H+ ions. In the present work, the corrosion rate of aluminium was assessed using weight loss and hydrogen evolution methods. The weight loss method is probably the most widely used method of corrosion inhibition assessment Citation44–47. The simplicity and reliability of the measurement offered by the weight loss method is such that the technique forms the baseline method of measurement in many corrosion monitoring programs Citation48. shows the plot of weight loss against time for Al corrosion in 1 M HCl without and with different concentrations of coconut coir dust extract (CCDE) at (a) 30 and (b) 60°C. Inspection of the figure reveals that the weight loss of aluminium specimens was reduced on introduction of CCDE into the corrosive medium.
Figure 2. Plot of weight loss against time for Al in 1 M HCl without and with different concentrations of CCDE at (a) 30°C and (b) 60°C.
The computed values of corrosion rate, inhibition efficiency, and degree of surface coverage from weight loss measurements are presented in . These parameters were computed using Equations (Equation4), (Equation5), and (Equation6), respectively.
Table 1. Calculated values of corrosion rate, inhibition efficiency and surface coverage for Al in 1 M HCl in the absence and presence of coconut coir dust extract at 30 and 60°C from weight loss measurements.
where CR is the corrosion rate, W is the weight loss of the aluminium coupon after 10 h of immersion (mg), ρ is the density of aluminium specimen (g cm−2), A is the area of the aluminium coupon (cm2) and t is the exposure time (h).
where CRblank and CRinh are the corrosion rates of the aluminium coupons in the absence and presence of CCDE respectively in 1 M HCl at the same temperature.
Results in the table indicate that the extract act as good corrosion inhibitor for aluminium in 1 M HCl solution given that the corrosion rate was reduced in the presence of the extract compared with its absence. Further inspection of reveals that corrosion rate increases with increase in temperature with the highest values obtained at 60°C both in the absence and presence of the extract.
It is seen from the data that the studied CCDE has inhibiting effect at the studied temperatures and the values of η(%) increases with increase in temperature. Thus, the acetone extract inhibitor efficiency was temperature-dependent. The fact that η(%) increases with temperature is explained by Ammar et al. Citation49 as the likely specific interaction between the aluminium surface and the inhibitor. Ivanov Citation50 considers the increase of η(%) with temperature increase as the change in the nature of the adsorption mode, the inhibitor is being physically adsorbed at lower temperatures, while chemisorption is favored as temperature increases. The data in the table also indicate that inhibition efficiency increases with increase in extract concentration. This can be attributed to an increase in the number of components of the extract adsorbed over the aluminiuml surface blocking the active sites in which direct acid attacks proceeds and protects the metal from corrosion.
The inhibition performances of CCDE could be explained as follows: FTIR spectrum in demonstrates that CCDE is a mixture of various compounds containing oxygen (O) and nitrogen (N) polar functions which all can be adsorbed on the corroded metal. The adsorption of components of CCDE onto the surface of Al alloy may take place through all these functional groups. As the corrosion resistant concentration increases, the area of the metal surface covered by the corrosion resistant molecule also increases, leading to an increase in the inhibition efficiency. One of the main criticisms of the use of plant extracts generally as corrosion inhibitors is the inability to pinpoint the major active component that is responsible for the corrosion inhibition effect owing to the complex chemical composition of the crude extracts. However, further investigation and the use of surface analytical techniques will enable the characterization of the active materials in the adsorbed layer and assist in identifying the most active ingredients in the acetone extract of Coconut Coir Dust which is currently being pursued in our laboratory.
2.3. Hydrogen evolution measurements
The following mechanisms can be proposed for hydrogen evolution reaction (HER) on electrodes in acidic media Citation51:
1. A primary discharge step (Volmer reaction)
2. An electrochemical-desorption step (Heyrowsky reaction)
3. A recombination step (Tafel reaction)
For hydrogen evolution reaction, the cathodic reaction may have three different steps: First, water molecule or hydronium ion is discharged on electrode surface to produce hydrogen atom in acidic solution and an adsorbed hydrogen atom, MHads, is generated (Volmer reaction). Second, one electron is transferred to a hydronium ion and the hydrogen evolution reaction occurs on metal surface (Heyrowsky reaction) or a pure chemical reaction takes place subsequently (Tafel reaction) Citation52.
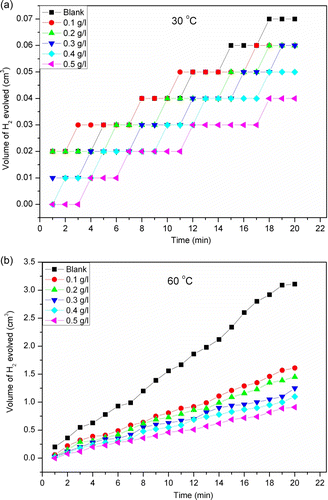
In spite of the three states for the formulation of the mechanism, no one of the three reactions formulated occurs as a single step but combines with another; i.e. Volmer reaction (slow) with the following Heyrowsky (faster) or Tafel (faster) reaction must be. If Volmer reaction is fast, Tafel and/or Heyrowsky reaction must be slow. The step of a slow reaction follows a fast step. So, the presence of CCDE extract may hinder the formation of MHad and suppress reaction (7) or hinder the electron transfer to H3O+ ion and suppress reaction (8). The corrosion rates of aluminium in the absence and presence CCDE were assessed by monitoring the volume of hydrogen gas evolved at fixed time intervals. shows representative plots of the volume of the hydrogen gas evolved as a function of reaction time at 30 and 60°C for Al in 1 M HCl in the absence and presence of different concentrations of the CCDE. Inspection of the figure shows a remarkable increase in the volume of H2 gas evolved in the blank acid solution at both temperatures studied. On the introduction of CCDE into the corrosive medium, it is seen that there is a considerable reduction in the volume of H2 gas evolved, suggesting that the components of CCDE were adsorbed onto the metal surface and blocked the electrochemical reaction efficiently by decreasing the available surface area.
Figure 3. Plot of volume of H2 evolved against time for Al in 1 M HCl without and with different concentrations of CCDE at (a) 30°C and (b) 60°C.
The rate of hydrogen evolution and inhibition efficiency was computed using Equations (Equation10) and (Equation11) respectively and the results are listed in .
Table 2. Calculated values of hydrogen evolution rate, inhibition efficiency and surface coverage for Al in 1 M HCl in the absence and presence of coconut coir dust extract at 30 and 60 °C from hydrogen evolution measurements.
where V t and V i are volumes of hydrogen evolved at time T t and T i, respectively.
where ρH (blank) and ρH (inh) are the rate of hydrogen evolution in the absence and presence of extracts, respectively.
The results show that the extracts diminished the H2 gas evolution rate and hence inhibited aluminium corrosion in the acidic environment. Generally the rate of H2 gas evolution decreased with increasing extract concentration, suggesting that the inhibiting action was concentration dependent. Also, the rate of H2 evolution was observed to increase with increase in temperature. Results presented in also show that inhibition efficiency increases with increase in the concentration of the extract and also with increase in temperature. Increase in inhibition efficiency with increase in temperature is suggestive of chemical adsorption of the CCDE components onto the aluminium surface. It is worthy to mention that inhibition efficiency obtained from the hydrogen evolution method follow the same trend observed from the weight loss method. However, the differences in results from the two independent methods can be attributed to differences in immersion time needed for the inhibiting species to get adsorbed and form a protective film on the aluminium surface thereby isolating the metal from attack of the aggressive anions present in solution. In addition, it has been argued that corrosion rate values from hydrogen evolution method represent instantaneous values while those one from weight loss method represent average values Citation53 Citation54.
2.4. Adsorption consideration
A number of mathematical relationships representing adsorption isotherms that characterize the dependence of the surface coverage function, θ, on the inhibitor concentration have been suggested to fit the various experimental data. Some of these isotherms are empirical and others are theoretical Citation55. An adsorbed molecule may make adsorption more difficult or less difficult if it is attached to another neighboring molecule. Thus, multilayer adsorption may take place through occupation of more than one surface site by an inhibitor molecule or the adsorption of more than one inhibitor molecule per surface site. A number of mathematical expressions have thus been developed to take into consideration some of the non-ideal effects. The most used expressions are Langmiur, Freundlich, Frumkin, Hill de Boer, Parsons, Temkin, Flory-Huggins, Bockris-Swinkels, adsorption isotherms, and kinetic-thermodynamic model Citation56. Basically, all the above isotherms having the form Citation57:
where f(θ, x) is the configurational factor which depends essentially on the physical model and assumptions underlying derivation of the isotherm, θ is the degree of surface coverage, a is the molecular interaction parameter depending on the molecular interactions in the adsorption layer and the degree of heterogeneity of the surface and C is the concentration. Moreover, all above expressions include the equilibrium constant of the adsorption process, K
ads, which is related to the standard free energy of adsorption by the expression:
where is the concentration of water expressed in g l−1 (the same as that of inhibitor concentration), R is the molar gas constant and T is the absolute temperature.
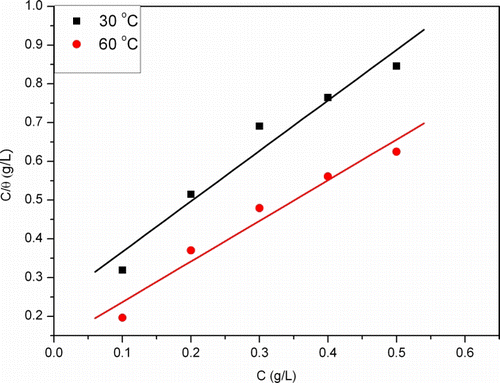
Attempt was made to fit the degree of surface coverage values to the earlier mentioned adsorption isotherm and the correlation coefficient R 2 was used to determine the best fit isotherm. By far, best result was obtained with Langmuir isotherm model. This isotherm is based on the assumption that all the adsorption sites are equivalent and that the particle-binding occurs independently from the nearby sites being occupied or unoccupied Citation58. It is characterized by:
shows the plot of vs C and linear plots were obtained at 30 and 60°C indicating that the adsorption of CCDE followed Langmuir isotherm. The various adsorption parameters obtained from this isotherm are listed in . The values of K
ads obtained from the Langmuir isotherm are listed in for the inhibitor (CCDE) as a function of temperature. It follows from the data listed on that the values of K
ads increases with temperature. These results confirm the suggestion that this inhibitor is chemically adsorbed and the strength of adsorption increases with temperature.
Table 3. Adsorption parameters from Langmuir isotherm for coconut coir dust extract in 1 M HCl for Al corrosion at 30 and 60°C.
The values of the free energy of adsorption, was obtained from Equation (Equation13) and are given in . Results presented in the table indicate that the values of
are negative in all cases and lies between −16.73 and −13.75 kJ/mol. The negative values signify a spontaneous adsorption of the inhibitor molecules and stability of the adsorption layer. Generally, values of
≤ −20 kJ mol−1 signify physisorption, and values more negative than −40 kJ mol−1 signify chemisorption Citation59
Citation60, physisorption is consistent with electrostatic interaction between charged molecules and a charged metal while chemisorption is consistent with charge sharing or transfer from the inhibitor components to the metal surface to form a co-ordinate type of bond. Although the increase in inhibition efficiency with increase in temperature suggests chemisorption of CCDE components onto aluminium surface, the values of free energy of adsorption seems to suggest otherwise. This discrepancy could be attributed to the fact that there are many limitations on the application of Langmuir adsorption isotherm for the analysis of inhibition phenomena and related data. According to Khaled and El-Maghraby Citation61, some of these limitations are that simple adsorption is applicable only under the following conditions:
| 1. | The surface of the metal is homogeneous. | ||||
| 2. | The adsorbate is specifically adsorbed, and each adsorbed species occupies only a single site of the surface. | ||||
| 3. | There is no surface diffusion of the adsorbed compounds. | ||||
| 4. | The standard free energy of adsorption is independent of the degree of coverage. | ||||
In addition, the adsorption may be highly selective with electrode reaction and may be potential-dependent, and the adsorbates may be competitive with water molecules.
The essential characteristic of Langmuir isotherm can be expressed in terms of a dimensionless separation factor, K L Citation62 Citation63 which describes the type of isotherm and is defined by:
The estimated values of K L for CCDE at different concentrations at 30 and 60°C are given in . If K L >1 unfavorable, K L =1 linear, 0 < K L <1 favorable, and K L =1 irreversible. It was found that all K L values are less than unity confirming that the adsorption process is favorable. However, according to the mean value of K L for each temperature, the inhibitory action of CCDE is more favorable at higher temperature.
Table 4. The values of dimensionless separation factor, K L , for coconut coir dust extract at various concentrations.
2.5. Effect of temperature
Temperature affects the rate of all electrochemical processes and influences adsorption equilibria and kinetics as well. Temperature investigations allow the determination of activation energy, pre-exponential factor and other thermodynamic activation functions in the absence and in the presence of inhibitor. The change of the corrosion process rate with the temperature increase was studied in 1 M HCl, both in the absence and in the presence of different concentrations of CCDE from weight loss measurements at 30 and 60°C.
The activation parameters for the corrosion process were calculated from Arrhenius-type plot according to the following equation:
where ρ2 and ρ1 are the corrosion rates at temperature T 1 and T 2, respectively, and R the molar gas constant. The calculated values of activation energy, E a are listed in . Inspection of the data show that the activation energy is lower in the presence of inhibitors than in its absence. The decrease in E a with CCDE concentration () is typical of chemisorption. This was attributed by Hoar and Holliday Citation64 to a slow rate of inhibitor adsorption with a resultant closer approach to equilibrium during the experiments at the higher temperature. But, Riggs and Hurd Citation65 explained that the decrease in activation energy of corrosion at higher levels of inhibition arises from a shift of the net corrosion reaction from that on the uncovered part on the metal surface to the covered one. Schmid and Huang Citation66 found that organic molecules inhibit both the anodic and cathodic partial reactions on the electrode surface and a parallel reaction takes place on the covered area, but that the reaction rate on the covered area is substantially less than on the uncovered area as obtained in the present work ( and ).
Table 5. Calculated values of activation energy E a and heat of adsorption Q ads for Al in 1 M HCl without and with different concentrations of coconut coir dust extract at 30–60°C.
An estimate of heat of adsorption was obtained from the trend of surface coverage with temperature as follows Citation67:
where θ1 and θ2 are the degrees of surface coverage at temperatures T 1 and T 2, The calculated values for both parameters are given in . The positive Q ads values indicate that the degree of surface coverage increased with rise in temperature, supporting the earlier proposed chemisorption mechanism for CCDE.
3. Experimental
Aluminium specimen used in the study was supplied by First Aluminium Company Nigeria Ltd and has the composition listed in . The sheet was 0.04 cm in thickness and was mechanically press-cut into 5×4 cm coupons. These were abraded with different grades (# 600, 800, 1000, and 1200) silicon carbide paper, degreased in absolute ethanol, dried in acetone, and stored in moisture-free desiccators prior to use. The corrosive medium was 1 M HCl prepared from 37% analytical grade supplied by Sigma-Aldrich. Deionised water was used for the preparation of all reagents.
Table 6. Elemental composition (Wt%) of the Al 3SR sample used in the study.
The coconut coir dust was obtained from local farmers and transported to the laboratory. One kilogramme of the coconut coir dust was extracted in Soxhlet apparatus using acetone for 48 h. The extract was concentrated initially using vacuum evaporator and finally by evaporation to dryness on a steam bath to obtain a solid residue devoid of acetone.
The apparatus used in the investigation included Perkin-Elmer FTIR spectrophotometer (100 series) for characterization of the acetone extract of the coir dust in order to determine the functional groups present in the extract that may be responsible to the corrosion inhibiting effect. The gasometric assembly, an apparatus that measures the volume of gas evolved from a reaction system. It consists of essentially two-necked round bottom flask which serves as the reaction medium containing the corrodent and the metal coupons. Others are a separating funnel, a burette fitted with taps and an outer glass jacket that serves as a water condenser.
Weight loss measurements were conducted in a glass reaction vessels containing 150 ml of test solution maintained at 30 and 60°C. Tests were performed under total immersion in the absence and presence of the inhibitor. In each experiment, the cleaned and weighed alumnum coupon was suspended in the different test solutions with the help of glass rod and hook. The test coupons were retrieved at 2 h interval progressively for 10 h, thoroughly cleaned as previously reported Citation67 and reweighed using digital analytical balance with sensitivity 0.1 mg. The weight loss was taken as the difference between the weight at a given time and the initial weight of the coupon. The tests were performed in triplicate to guarantee the reliability of the results and the mean value of the weight loss is reported. The reproducibility of the experiment was higher than 95%.
The procedure followed for the hydrogen evolution measurements have been described elsewhere Citation24 Citation31–33. The test solution was kept at 100 ml. The progress of corrosion in the absence and presence of test inhibitor was monitored by careful measurements of the volume of hydrogen gas evolved at fixed time intervals. The experiments were performed for 1 M HCl (blank) and different concentrations of CCDE (0.1–0.5 g/l) at 30 and 60°C maintained using a thermostated water bath.
4. Conclusions
Corrosion inhibitive effect of CCDE for Al in 1 M HCl has been assessed using chemical techniques of corrosion monitoring at 30 and 60°C. Results obtained showed that CCDE acts as a good corrosion inhibitor for acid induced corrosion of Al. Inhibition efficiency increases with increase in CCDE concentration and also with increase in temperature. The inhibition was assumed to occur via adsorption of the CCDE components on the metal surface, which depends on the molecular structure and functional groups present on the extract. The adsorption of the extract on the Al surface obeys the Langmuir adsorption isotherm. Chemical adsorption mechanism is proposed from the trend of inhibition efficiency with temperature which is also corroborated by kinetic and thermodynamic parameters obtained.This present study provides new information on the inhibiting characteristics of CCDE under specified conditions. The environmental friendly inhibitor could find possible applications in metal surface anodizing and surface coating in industries.
References
- Rosliza , R. ; Wan Nik , W.B. ; Senin , H.B. Mater. Chem. Phys . 2008 , 107 , 281 – 288 .
- Shreir , L.L. ; Jarman , R.A. ; Burstein , G.T. Metal/Environmental Reactions, Corrosion , 3rd ed Oxford : Butterworth Heinemann Press , 1994 ; 1 , 1 – 16 .
- Pourbaix , M. Atlas of electrochemical equilibria in aqueous solutions ; New York : Pergamon Press ; 1966 .
- Aytac , A. J. Mater. Sci . 2010 , 45 , 6812 – 6818 .
- Obot , I.B. ; Obi-Egbedi , N.O. Colloids Surf A: Physicochem. Eng. Asp . 2008 , 330 , 207 – 212 .
- Obot , I.B. ; Obi-Egbedi , N.O. ; Umoren , S.A. Corros. Sci . 2009 , 51 , 1868 – 1875 .
- Abd El Rehim , S.S. ; Hassan , H.H. ; Amin , M.A. Mater. Chem. Phys . 2002 , 78 , 337 – 348 .
- Maayta , A.K. ; Al-Rawashdeh , N.A.F. Corros. Sci . 2004 , 46 , 1129 – 1140 .
- Foad El-Sherbini , E.E. ; Abd-El-Wahab , S.M. ; Deyab , M.A. Mater. Chem. Phys . 2003 , 82 , 631 – 637 .
- Zhang , Q. ; Hua , Y. Mater. Chem. Phys . 2010 , 119 , 57 – 64 .
- Ashassi-Sorkhabi , H. ; Shabani , B. ; Aligholipour , B. ; Seifzadeh , D. Appl. Surf. Sci . 2006 , 252 , 4039 – 4047 .
- Kàlmàn , E. Inhibitors of low toxicity for aqueous solution . In Proceedings of 7th European symposium on corrosion inhibitors (7SIEC) Ferrara , Italy , 17–21 Sebtember 1990 .
- Ekanem , U.F. ; Umoren , S.A. ; Udousoro , I.I. ; Udoh , A.P. J. Mater. Sci . 2010 , 45 , 5558 – 5566 .
- Bouyanzer , A. ; Hammouti , B. ; Majidi , L. Mater. Lett . 2006 , 60 , 2840 – 2484 .
- Chauhan , L.R. , Gunasekaran , G. Corros. Sci . 2007 , 49 , 1143 – 1161 .
- El-Etre , A.Y. Appl. Surf. Sci . 2006 , 252 , 8521 – 8525 .
- El-Etre , A.Y. Mater. Chem. Phys . 2008 , 108 , 278 – 282 .
- El-Etre , A.Y. J. Colloid Interf. Sci . 2007 , 314 , 578 – 583 .
- Umoren , S.A. ; Obot , I.B. ; Obi-Egbedi , N.O. J. Mater. Sci . 2009 , 44 , 274 – 279 .
- Radojcic , I. ; Berkovic , K. ; Kovac , S. ; Vorkapic-Furac , J. Corros. Sci . 2008 , 50 , 1498 – 1504 .
- Rahim , A.A. ; Rocca , E. ; Steinmetz , J. ; Kassim , M.J. ; Adnan , R. ; Ibrahim , M.S. Corros. Sci . 2007 , 49 , 402 – 417 .
- Fu , J.J. ; Li , S.N. ; Cao , L.H. ; Wang , L. ; Yan , L.H. ; Lu , L.D. J. Mater. Sci . 2010 , 45 , 979 – 986 .
- Obot , I.B. ; Obi-Egbedi , N.O. ; Umoren , S.A. Arab. J. Chem . 2010 . doi: 10.1016/j.arabjc.2010.09.002
- Ating , E.I. ; Umoren , S.A. ; Udousoro , I.I. ; Ebenso , E.E. ; Udoh , A.P. Green Chem. Lett. Rev . 2010 , 3 , 61 – 68 .
- Umoren , S.A. ; Obot , I.B. ; Ebenso , E.E. ; Obi-Egbedi , N.O. Desalination 2009 , 247 , 561 – 572 .
- Umoren , S.A. ; Obot , I.B. ; Ebenso , E.E. ; Obi-Egbedi , N.O. Portug. Electrochim. Acta 2008 , 26 , 199 – 209 .
- Umoren , S.A. Cellulose 2008 , 15 , 751 – 761 .
- Umoren , S.A. ; Obot , I.B. ; Ebenso , E.E. ; Okafor , P.C. ; Ogbobe , O. ; Oguzie , E.E. Anti-corros. Methods Mater . 2006 , 53 , 277 – 282 .
- Tejano , E.A. State of the Art of Coconut Coir Dust and Husk Utilization (General Overview) , Paper presented during the National Workshop on Waste Utilization, Coconut Husk held on November 12 , at the Philippine Coconut Authority, Diliman, Quezon City , Phillipines , 12–14 November 1984 .
- Gonzales , B.P. Analysis and Pulping of Coir Dust ; Emata , R. Coconut Research and Development , 3 ; Manila : UCAP , 1970 ; 163 – 173 .
- Joachim , A.W.R. Chem. Abst . 1930 , 24 , 1796 .
- Meerow , A.W. Coir Dust, A Viable Alternative To Peat Moss, Greenhouse . Product News , 15 January , 1997 , 17 – 21 .
- Vidhana Arachchi , L.P. ; Somasiri , L.L.W. Cocos . 1997 , 12 , 54 – 60 .
- Reynolds , S.G. Acta Hortic . 1974 , 37 , 1983 – 1989 .
- Chweya , J.A. ; Gurnah , A.M. ; Fisher , N.M. East Afr. Agric. For. J . 1978 , 43 , 334 – 339 .
- Offord , C.A. ; Muir , S. ; Tyler , J.L. Aust. J. Exp. Agric . 1998 , 38 , 879 – 887 .
- Noguera , P. ; Abad , M. ; Noguera , V. ; Puchades , R. ; Maquieira , A. Acta Hortic . 2000 , 51 , 279 – 285 .
- Oguzie , E.E. Corros Sci . 2001 , 49 , 1527 – 1539 .
- El-Etre , A.Y. Corros. Sci . 2003 , 45 , 2485 – 2495 .
- Abdel-Gaber , A.M. ; Khamis , E. ; Abo-ElDahab , H. ; Adeel , Sh. Mater. Chem. Phys . 2008 , 109 , 297 – 305 .
- Abiola , O.K. ; Otaigbe , J.O.E. Corros. Sci . 2009 , 51 , 2790 – 2793 .
- Abiola , O.K. ; Otaigbe , J.O.E. ; Kio , O.J. Corros. Sci . 2009 , 51 , 1879 – 1881 .
- Satapathy , A.K. ; Gunasekaran , G. ; Sahoo , S.C. ; Amit , K. ; Rodrigues , P.V. Corros. Sci . 2009 , 51 , 2848 – 2856 .
- Musa , Y. ; Khadom , A.A. ; Kadhum , A.H. ; Mohamad , A.B. ; Takriff , M.S. J. Taiwan Ins. Chem. Engr . 2010 , 41 , 126 – 128 .
- Khadom , A.A. ; Yaro , A.S. ; Kadum , A.H. J. Taiwan Ins. Chem. Engr . 2010 , 41 , 122 – 125 .
- Bouklah , M. ; Hammouti , B. ; Lagrenee , M. ; Bentiss , F. Corros. Sci . 2006 , 48 , 2831 – 2842 .
- Chitra , S. ; Parameswari , K. ; Sivakami , C. ; Selvaraj , A. Chem. Eng. Res. Bull . 2010 , 14 , 1 – 6 .
- Afidah , A.R. ; Kassim , J. Recent Patents on Mater. Sci . 2008 , 1 , 223 .
- Ammar , I.A. ; El Khorafi , F.M. Werkst. Korros . 1973 , 24 , 702 – 709 .
- Ivanov , E.S. Inhibitors for Metal Corrosion in Acid Media , Metallurgy , Moscow , 1986 .
- Aydin , R. ; Koleli , F. Prog. Org. Coat . 2006 , 56 , 76 – 80 .
- Bhardwaj , M. ; Balasubramaniam , R. Int. J. Hydrogen Energy 2008 , 33 , 2178 – 2188 .
- Umoren , S.A. ; Ogbobe , O. ; Ebenso , E.E. ; Okafor , P.C. J. Appl. Polym. Sci . 2007 , 105 , 3363 – 3370 .
- Umoren , S.A. J. Appl. Polym. Sci . 2011 , 119 , 2072 – 2084 .
- Mazhar , A.A. ; Salih , S.A. ; Gad-Allah , A.G. ; Tammam , R.H. J. Mater. Eng. Perf . 2008 , 17 , 260 – 270 .
- El-Awady , A. ; Abd El-Nabey , B. ; Aziz , G. J. Electrochem. Soc . 1992 , 139 , 2149 – 2154 .
- Khamis , E. ; Bellucci , F. ; Latanision , R. ; El-Ashry , E. Corrosion 1991 , 47 , 677 – 681 .
- Rosliza , R. ; Wan Nik , W.B. Curr. Appl. Phys . 2010 , 10 , 221 – 229 .
- Alberty , R. ; Silbey , R. Physical Chemistry , 2nd ed New York : Wiley , 1997 ; 845 .
- Amin , M.A. ; Ibrahim , M.M. Corros. Sci . 2011 , 53 , 873 – 885 .
- Khaled , K.F. ; El-Maghraby , A. Arab. J. Chem . 2010 . doi: 10.1016/j.arabjc.2010.11.005
- Noor , E.A. Mater. Chem. Phys . 2009 , 114 , 533 – 541 .
- Obot , I.B. ; Obi-Egbedi , N.O. J. Appl. Electrochem . 2010 , 40 , 1977 – 1984 .
- Hoar , T.P. ; Holliday , R.D. J. Appl. Chem . 1953 , 3 , 502 – 509 .
- Riggs , L.O. Jr. ; Hurd , T.J. Corrosion 1967 , 23 , 252 – 262 .
- Schmid , G.M. ; Huang , H.J. Corros. Sci . 1980 , 20 , 1041 – 1057 .
- Obot , I.B. ; Umoren , S.A. ; Obi-Egbedi , N.O. J. Mater. Environ. Sci . 2011 , 2 , 60 – 71 .