Abstract
An efficient and environmentally benign procedure is developed for the synthesis of 1,5-benzothiazepine and 1,2,4-triazolo[3,4-b][1,3,4] thiadiazepine derivatives in good to excellent yield under solvent-free microwave irradiations using silica sulfuric acid or basic alumina respectively as solid supports.
Introduction
1,5-benzothiazepine moiety is a privileged class of pharmacophore, as compounds bearing this structural unit possess a broad spectrum of biological activities such as anti-convulsant, Citation1 Ca2+ channel antagonist Citation2 Citation3, anti-anginal Citation4, anti-HIV Citation5, squalene synthetase inhibitor Citation6, V2 arginine vasopressin receptor antagonist Citation7, HIV-1 reverse transcriptase inhibitor Citation8, etc.
Despite their importance from a pharmacological and synthetic point of view, few methods for the preparation of 1,5-benzothiazepines are reported in the literature. These include‘e the reaction of 1,3-diarylprop-2-enones (chalcones) with 2-aminothiophenol Citation9. The various reported methodologies involve the use of inorganic supports at 80 °C for 3 hours Citation10, AcOH or DMF under microwave irradiation Citation11, AcOH or TFA in EtOH or toluene under refluxCitation12–14, AcOH in DMF or EtOH at 60 °C for 5 hours followed by keeping at room temperature for overnight Citation15, EtOH saturated with HCl under reflux for 3 hoursCitation16, piperidine in toluene under reflux for 8 hours, and pyridine under reflux for 3 hoursCitation17 Citation18. However, these methodologies have one or more disadvantages such as the use of high boiling solvent that is difficult to recover, excess amounts of acid or base and using corrosive materials (e.g., HCl gas, TFA), etc.
Recently, an environmentally benign synthetic approach for 1,5-benzothiazepines derivatives have been reported Citation19 with microwave irradiation under solvent and catalyst free conditions, but the later mentioned procedure has some limitations such as low yield of reaction.
Thus, there is a necessity to develop more and more benign effective methods for the synthesis of derivatives of 1,5-benzothiazepines for biological screening purposes. Solid supported reagents have made a landmark and made significant contributions to preserve the green environment by reducing the waste effluent Citation20. with the development of microwave ovens, it gives remarkable rate enhancement, higher yields, greater selectivity and ease of manipulation. Motivated by the afore-mentioned findings and our ongoing endeavours Citation21–23 in the development of environmentally benign protocols, we now describe a microwave accelerated solid state approach for the rapid assembly of substituted thiazepine and thiadiazepine rings.
Results and discussion:
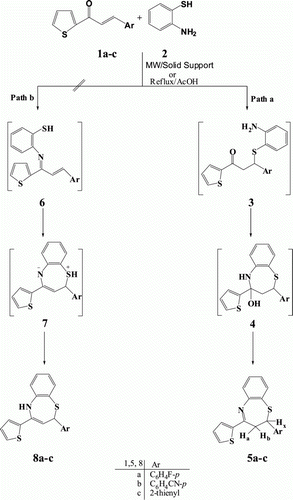
The reaction of chalcones 1a-c with o-aminothiophenol (2) in the presence of silica-sulfuric acid was carried out without solvent under microwave irradiations afforded 2-aryl-2,3-dihydro-4-(thiophene-2-yl)-1,5-benzothiazepine derivatives 5a-c within 60–120 second as evidenced by TLC (), while the same reaction carried out via reflux in acetic acid required 10-12 h ().
Table 1. Synthesis of 2,3-dihydro-1,5-benzothiazepines 5a-c under conventional (reflux) condition and microwave irradiations.
The construction of the 1,5-benzothiazepine moiety from 1a-c and o-aminothiophenol (2) may involve two pathways: (a) conjugate addition of the sulfhydryl group of 2 to the α,β-unsaturated carbonyl group of 1a-c leading to the intermediate formation of the thia-Michael adduct 3 which, on subsequent intramolecular nucleophilic attack by the NH2 group on the carbonyl carbon followed by dehydration, forms the 2,3-dihydro-1,5-benzothiazepine 5a-c (Path a) or (b) condensation of the amino group of 2 with the carbonyl group of 1a-c leading to the intermediate formation of the aza-diene 6, which on subsequent intramolecular conjugate addition by the sulfhydryl group forms the isomeric 2,5-dihydro-1,5-benzothiazepine 8a-c (Path b) Citation24 ().
The reaction products 5a-c that assumed to be formed via path a () were identified by their analytical and spectral data. The other possible isomeric structures 8a-c were excluded due to absence of NH band in the IR spectrum of reaction products and their 1H NMR spectra reveals no exchangeable signals due to NH proton. The structures of compounds 5a-c were established on the basis of their spectroscopic data. The IR spectra of condensed products displayed disappearance of band at 1650–1660 cm −1 due to C=O of chalcones and –SH of o-aminothiophenol (2) at 2570 cm−1 and appearance of a band at 1585–1602 cm−1 due to C=N. summarize the time of reactions, yields and m.p of products under both conventional condition (reflux in acetic acid) and microwave irradiations.
shows that solvent free synthesis of 1,5-benzothiazepine under microwave irradiations reduced the time of reactions from several hours to minutes and improved the yields from 59-65% (under conventional conditions) to 82-89%.
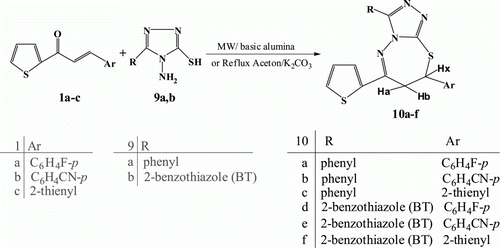
In a similar manner, 1-amino-2-mercapto-5-substituted triazoles 9a,b reacted with chalcones 1a-c on basic alumina under microwave irradiations to afford the 7,8-dihydro-3,7-diaryl-9-(thiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazepine derivatives 10a-f () which was obtained in excellent yield and shorter reaction time in comparing with conventional condition (reflux in acetone in presence of K2CO3) as shown in .
Table 2. Synthesis of 7,8-dihydro[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepines10a-f under microwave irradiations.
The structure of,8-dihydro[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepines derivatives 10a-f was assigned on the basis of their elemental analyses and spectral data, for example, the 1H NMR spectrum of compound 10a revealed three dd signals at δ 3.52, 3.94 and 4.48 due to Ha, Hb and Hx protons respectively in addition to aromatic multiplet and thiophene protons at δ 6.95–7.65. The mass spectrum of the same compound revealed a peak corresponding to its molecular ion at m/z 406.
To find optimum conditions for microwave assisted solvent free synthesis of 1,5-benzothiazepines 5a-c and 7,8-dihydro[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepines 10a-f, the above two reactions were studied under varying different inorganic solid supports. The results were summarized in .
Table 3. Inorganic solid supports effect on time and yielda in the synthesis of compounds 5a, 10a, 10d.
The results in indicates that (i) Silica sulfuric acid was the most effective among the solid supports tested to promote the reaction of chalcones 1a with o-aminothiophenol (2) with highest yield in shortest time. (ii) Basic alumina was the most effective solid support to promote the reaction of 1-amino-2-mercapto-5-substituted triazoles 9a,b and chalcones 1a in shortest time and highest yield. All reaction times were determined by follow the reaction progress via thin layer chromatography (TLC).
The effect of microwave irradiation power in this reaction was also investigated. The results show that the highest yield of compound 5a is obtained at a power of 800 W (). Hence, it's better for the reaction to be carried out at 800 W power settings.
Table 4. The effect of microwave irradiation power on the formation of 5a a .
Also, the effect of irradiation time on the reaction was studied and the results summarized in at Irradiation power is 800 watts.
Table 5. The effect of microwave irradiation time on the formation of 5a a .
From , it is obvious that when the reaction was carried out under microwave irradiation for 1 min. highest yield of 5a was obtained, However, no further improvement of the yield was noted when the reaction time was prolonged to 2 or 3 min. and the yield even decreased a little, a fact we attribute to the formation of byproducts.
Finally, microwave irradiations assisted solvent free synthesis of 1,5-benzothiazepines 5a-c and 7,8-dihydro[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepines 10a-f, on inorganic solid supports such as alumina or silica sulfuric acid. This method gives faster reactions, higher yields with simplified separation, these may be due to good dispersion of active site (reagent) which leads to significant improvement of reactivity–large surface area. All reactions were performed in the appropriate volume vessel with temperature monitoring.
In conclusion, a facile rapid, economic and more benign effective methodology has developed for the synthesis of new derivatives of 1,5-benzothiazepines and [1,2,4]triazolo[3,4-b][1,3,4]-thiadiazepines containing thiophene moiety on a solid supports under the influence of microwave irradiations, in the absence of any toxic and mineral acids, bases or organic solvent.
EXPERIMENTAL
Melting points were measured on a Gallenkamp melting point apparatus and are uncorrected. The infrared spectra were recorded in potassium bromide disks on a pye Unicam SP 3300 and Shimadzu FTIR 8101 PC infrared spectrophotometers with adsorptions in cm−1. The 1H-NMR spectra were recorded on a Varian Mercury VXR-300 NMR spectrometer. 1H spectra were run at 300 MHz in deuterated chloroform (CDCl3) or dimethyl sulphoxide (DMSO-d6). Chemical shifts were related to that of the solvent and expressed in ppm downfield from internal standard tetramethylsilane. Mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX at 70 e.V and GC/MS finnigan SSQ 7000 spectrophotometers. Elemental analyses were carried out at the Microanalytical Center of Cairo University, Giza, Egypt.
Microwave assisted reactions were performed using modified Amana domestic microwave oven (2450 MHZ, 800 W) under atmospheric pressure, temperature measurement is performed for all reactions with fiber optic sensors, to monitor the temperature inside the vessel, it was found that≈105–110 °C. It is important to mention that using of domestic microwave in chemical reactions is potentially hazardous due to lack of safety. Therefore, it should be using only microwave reactors for chemical reactions.
The purity of compounds was checked on silica gel coated aluminum plates. Silica sulfuric acidCitation25, chalcones 1a-c Citation26 and 1-amino-2-mercapto-5-aryl-1,3,4-triazoles 9a,b Citation27 were prepared according to the reported literature.
General procedure for synthesis of 2-Aryl-2,3-dihydro-4-(thiophen-2-yl)benzo[1,5]thiazepines 5a-c:
Method A:
A mixture of chalcones 1a, 1b or 1c (20 mmol) and o-aminothiophenol (2) (24 mmol) in glacial AcOH (20 mL) was refluxed for suitable time (as examined by TLC, see ). The reaction mixture was then concentrated under vaccum and resulting precipitate was filtered, washed and recrystallized from ethanol.
Method B:
Silica sulfuric acid (1 g), was added to chalcones 1a-c (20 mmol) and o-aminothiophenol (2) (24 mmol). The reaction mixture was mixed by grinding in a morter and placed in an Erlenmeyer flask inside the microwave oven then irradiated at 800watt power for a suitable time () with an interval 0.5 min. The mixture was cooled and the product was extracted with Ethanol/DMF (1:1). After evaporating the volatile materials by vacuum, compounds 5a-c were filtered and crystallized from ethanol.
2,3-dihydro-2-(4-Fluorophenyl)-4-(thiophen-2-yl)benzo[1,5]thiazepine (5a)
The pure product was obtained as white powder. IR ν: 1585 (C=N) cm−1, 1H NMR (CDCl3): δ 2.21 (dd, 1H, J=12.3,10.1 Hz), 3.95 (dd, 1H, J=12.3, 3.4 Hz), 5.01 (dd, 1H, J=10.1, 3.3 Hz), 7.13–7.48 (m, 11H, ArH and thiophene protons). Ms (m/z): 339(M+). Anal. Calcd. for C19H14FNS2: C, 67.23; H, 4.16; N, 4.13; S, 18.89. Found: C, 67.33; H, 4.21; N, 4.04; S, 18.83.
2-(4-Cyanophenyl)-2,3-dihydro-4-(thiophen-2-yl)benzo[1,5]thiazepine (5b)
The pure product was obtained as dark grey powder. IR ν: 2217 (C≡N), 1598 (C=N) cm−1, 1H NMR (CDCl3): δ 2.39 (dd, 1H, J=11.8,9.6 Hz), 4.01 (dd, 1H, J=11.8, 3.9 Hz), 5.52 (dd, 1H, J=9.6, 4.00 Hz), 6.59–7.78 (m, 11H, ArH and thiophene protons). Ms (m/z): 346(M+). Anal. Calcd.for C20H14N2S2: C, 69.33; H, 4.07; N, 8.09; S, 18.51. Found: C, 69.40; H, 4.11; N, 8.07; S, 18.42%
2,3-Dihydro-2,4-di(thiophen-2-yl)benzo[1,5]thiazepine (5c)
The pure product was obtained as dark grey powder. IR ν:: 1603 (C=N) cm−1, 1H NMR (CDCl3): δ 2.7 (dd, 1H, J=12.6,10.7 Hz), 4.25 (dd, 1H, J=12.6, 3.3 Hz), 5.22 (dd, 1H, J=10.7, 3.3 Hz), 7.00–7.35 (m, 10H, ArH and thiophene protons). MS (m/z): 327(M+). Anal. Calcd. for C17H13NS3: C, 62.35; H, 4.00; N, 4.28; S, 29.37. Found: C, 62.45; H, 4.10; N, 4.17; S, 29.28.
General procedure for the synthesis of 3,8-diaryl-7,8-Dihydro-6-(thiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine derivatives 10a-f:
Method A:
To a solution of substituted 1,2,4-triazoles 9a or 9b (20 mmol) in acetone (10 ml), chalcones 1a, 1b or 1c (20 mmole) and 1g of K2CO3 were added. The reaction mixture was refluxed for appropriate time (as examined by TLC, see ). The reaction mixture was cooled, inorganic salt was filtered off and solvent was evaporated. The solid obtained was recrystalized from ethanol/DMF (1:1).
Method B:
Basic alumina (1 g) was added to the 1a-c (20 mmol) and 1-amino-2-mercapto-5-aryl-1,3,4-triazole 9a,b (20 mmol) and mixed by grinding in a morter, placed in an Erlenmeyer flask inside the microwave oven then irradiated at 800 watt power for a suitable time (). The mixture was cooled and the product was extracted with ethyl acetate 10a-c or ethanol 10 d-f. The products were collected by evaporating the solvent and recrystallized from ethanol/DMF (1:1).
7,8-dihydro-8-(4-Fluorophenyl)-3-phenyl-6-(thiophen-2-yl)-[1,2,4]triazolo-[3,4-b][1,3,4]thiadiazepine (10a)
The pure product was obtained as colorless crystal. IR ν:: 1598 (C=N) cm−1, 1H NMR (DMSO-d6): δ 3.52 (dd, 1H, J=11.8, 9.2 Hz), 3.94 (dd, 1H, J=11.8, 4.3 Hz), 4.48 (dd, 1H, J= 9.2, 4.3 Hz), 6.95-7-56 (m, 12H, ArH and thiophene protons), MS (m/z): 406(M+). Anal. Calcd. for C21H15FN4S2: C, 62.05; H, 3.72; N, 13.78; S, 15.78. Found: C, 62.11; H, 3.76; N, 13.73; S, 15.73.
8-(4-Cyanophenyl)-7,8-dihydro-3-phenyl-6-(thiophen-2-yl)-[1,2,4]triazolo-[3,4-b][1,3,4]thiadiazepine (10b)
The pure product was obtained as colorless crystal IR ν:: 2204 (C≡N), 1605 (C=N) cm−1, 1H NMR (DMSO-d6): δ 3.29 (dd, 1H, J=10.4, 9.4 Hz), 4.03 (dd, 1H, J=10.4, 5.6 Hz), 4.44 (dd, 1H, J=9.4, 5.6 Hz), 7.19–7.87 (m, 12H, ArH's and thiophene protons), MS (m/z): 413(M+). Anal. Calcd. For C22H15N5S2: C, 63.90; H, 3.66; N, 16.94; S, 15.51. Found: C, 64.02; H, 3.69; N, 16.88; S, 15.42.
7,8-Dihydro-6,8-di(thiophen-2-yl)-3-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]-thiadiazepine (10c)
The pure product was obtained as colorless crystal, IR ν:: 1605 (C=N) cm−1, 1H NMR (DMSO-d6): δ 3.35 (dd, 1H, J=11.00, 8.9 Hz), 4.03 (dd, 1H, J=11.00, 6.1 Hz), 4.44 (dd, 1H, J=8.9, 6.1 Hz), 6.29–7.36 (m, 11H, ArH and thiophene protons), MS (m/z): 394(M+). Anal. Calcd. for C19H14N4S3: C, 57.84; H, 3. 58; N, 14.20; S, 24.38. Found: C, 57.90; H, 3.63; N, 14.17; S, 24.30.
3-(Benzo[d]thiazol-2-yl)-7,8-dihydro-8-(4-fluorophenyl)-6-(thiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine (10d)
The pure product was obtained as colorless crystal. IR ν:: 1585 (C=N) cm−1, 1H NMR (DMSO-d6): δ 3.05 (dd, 1H, J=12.7, 4.4 Hz, Ha), 3.32 (dd, 1H, J=12.7, 6.52 Hz, Hb), 4.44 (dd, 1H, J=6.52, 4.5 Hz, Hx, CH), 7.22–7.51 (m, 11H, ArH and thiophene protons), MS (m/z): 463(M+). Anal. Calcd. For C22H14FN5S3: C, 57.00; H, 3.04; N, 15.11; S, 20.75. Found: C, 57.09; H, 3.12; N, 15.01; S, 20.68.
3-(Benzo[d]thiazol-2-yl)-8-(4-cyanophenyl)-7,8-dihydro-6-(thiophen-2-yl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazepine (10e)
The pure product was obtained as colorless crystal. IR ν:: 2193 (C≡N),1595 (C=N) cm−1, 1H NMR (DMSO-d6): δ 2.72 (dd, 1H, J=13.00, 5.3 Hz), 3.02 (dd, 1H, J=13.2, 6.9 Hz), 3.82 (dd, 1H, J=6.9, 5.3 Hz), 7.20–8.1 (m, 11H, ArH and thiophene protons), MS (m/z): 470(M+). Anal. Calcd. For C23H14N6S3: C, 58.70; H, 3.00; N, 17.86; S, 20.44. Found: C, 58.80; H, 3.06; N, 17.82; S, 20.32.
3-(benzo[d]thiazol-2-yl)-7,8-dihydro-6,8-di(thiophen-2-yl)-[1,2,4]triazolo-[3,4-b][1,3,4]thiadiazepine (10f)
The pure product was obtained as colorless crystal. IR ν:: 2193 (C≡N),1595 (C=N) cm−1, 1H NMR (DMSO-d6): δ 3.06 (t, 1H, J=12.8 Hz), 3.30 (dd, 1H, J=12.8, 4.9 Hz), 4.98 (dd, 1H, J=12.8, 5.00 Hz), 6.99–7.52 (m, 10H, ArH and thiophene protons), MS (m/z): 451(M+). Anal. Calcd. For C20H13N5S4: C, 53.19; H, 2.90; N, 15.51; S, 28.40. Found: C, 53.27; H, 2.49; N, 15.48; S, 28.31.
References
- Sarro , G. D. ; Chimirri , A. ; Sarro , A. D. ; Gitto , R. ; Grasso , S. ; Zappalá , M. Eur. J. Med. Chem . 1995 , 30 , 925 .
- Bariwal , J. B. ; Upadhyay , K. D. ; Manvar , A. T. ; Trivedi , J. C. ; Singh , J. S. ; Jain , K. S. ; Shah , A. K. ; European Journal of Medicinal Chemistry 2008 , 43 , 2279 .
- Kurokawa , J. ; Adachi-Akahane , S. ; Nagao , T. Eur. J. Pharmacol . 1997 , 325 , 229 .
- Miyata , O. ; Tetsuro , S. ; Ichiya , N. ; Takeaki , N. Tetrahedron 1997 , 53 , 2421 .
- Grandolini , G. ; Perioli , L. ; Ambrogi , V. Eur. J. Med. Chem . 1999 , 34 , 701 .
- Yang , X. ; Buzon , L. ; Hamanaka , E. ; Liu , K. K.-C. Tetrahedron: Asymmetry 2000 , 11 , 4447 .
- Urbanski , M. J. ; Chen , R. H. ; Demarest , K. T. ; Gunnet , J. ; Look , R. ; Ericson , E. ; Murray , W. V. ; Rybczynski , P. J. ; Zhang , X. Bioorg. Med. Chem. Lett . 2003 , 13 , 4031 .
- Di Santo , R. ; Costi , R. Farmaco 2005 , 60 , 385 .
- Lévai , A. J. Heterocycl. Chem . 2000 , 37 , 199 .
- Kodomari , M. ; Noguchi , T. ; Aoyama , T. Synth. Commun . 2004 , 34 , 1783 .
- Patel , V. M. ; Desai , K. R. Indian J. Chem. Sect. B 2004 , 43 , 199 .
- Lévai , A. J. Heterocycl. Chem . 2004 , 41 , 399 .
- Lévai , A. J. Heterocycl. Commun . 2002 , 8 , 227 .
- Lévai , A. J. Heterocycl. Commun . 2002 , 8 , 375 .
- Micheli , F. ; Degiorgis , F. ; Feriani , A. ; Paio , A. ; Pozzan , A. ; Zarantonello , P. ; Seneci , P. J. Comb. Chem . 2001 , 3 , 224 .
- Pant , S. ; Singhal , B. ; Upreti , M. ; Pant , U. Molecules 1998 , 3 , 159 .
- Upreti , M. ; Pant , S. ; Dandia , A. ; Pant , U. C. Indian J. Chem. Sect. B: Org. Chem. Incl. Med. Chem . 1997 , 36 , 1181 .
- Pant , S. ; Sharma , A. ; Sharma , S. K. ; Pant , U. C. ; Goel , A. K. Indian J. Chem. Sect. B: Org. Chem. Incl. Med.Chem . 1996 , 35 , 794 .
- Rahman , M. ; Roy , A. ; Majee , A. ; Hajra , A. J. Chem. Res . 2009 , 178 .
- Kidwai , M. ; Sapra , P. ; Misra , P. ; Saxena , R. K. ; Singh , M. Bioorganic & Medicinal Chemistry 2001 , 9 , 217 .
- Saleh , T.S ; Abd EL-Rahman N. M. ; Ultrasonics Sonochemistry , 2009 , 16 , 237 .
- Abd EL-Rahman N. M. ; Saleh , T.S ; Mady , M.F. Ultrasonics Sonochemistry , 2009 , 16 , 70 .
- Abd El Maksod , I. H. ; Saleh , T. S. ; Green chemistry Letters and reviews , 2009 , Accepted For Publication .
- Gupta , M. ; Paul , S. ; Gupta , R. Indian J. Chemistry, Sec. B 2009 , 48B , 460 .
- Zolfigol , M. A. Tetrahedron 2001 , 57 , 9509 .
- El-Rayyes , N.R. , Journal of heterocyclic chemistry , 1982 , 19 , 415 .
- Eisa , H. M. ; El-Emam , A. A. ; Moustafa , M. A. ; El-Kerdawy , M. M. J. Chin. Chem. Soc . 1988 , 35 , 393 .