Abstract
A convenient, eco-friendly, and efficient method for synthesis of bis(3-indolyl)methanes by the reaction of indoles with various aromatic aldehydes “on-water” has been developed. The attractive features of this method are that in cases of aldehydes which are easily oxidized to acids, no external catalyst is necessary while in other cases a trace amount (ca. 5 mol%) of commercially available and inexpensive catalyst benzoic acid is sufficient to give good to excellent yield of products.
Introduction
A good number of indole derivatives find important applications in the fields of pharmaceuticals, agrochemicals, and material sciences Citation1–3, which have made synthesis of indole derivatives an important research area for organic chemists. Among the indole derivatives, bis(3-indolyl)methanes (BIMs) exihibit a wide range of biological activities such as antimicrobial and antifungal Citation4, antibacterial Citation5, analgesic Citation6, cytotoxic Citation7, and anti-inflammatory Citation6 activities. Cancer chemotherapy with BIMs has recently been reviewed Citation8. BIMs inhibit bladder cancer growth Citation9, mammary tumor growth Citation10, and the proliferation process of breast tumor cells Citation11. Due to the versatile possibilities of application of BIMs, there is a continuous quest for more efficient, economic, and environment-friendly methods for their synthesis.
Indole can readily undergo electrophilic substitution reaction with carbonyl compounds to form BIMs. Different protic acids, such as HCl, H2SO4, CH3COOH, HCOOH, amberlyst, silica sulfuric acid (SSA), and p-TsOH, and Lewis acids, such as InCl3, In(OTf)3, Ln(OTf)3, LiClO4, VCl3, CuBr2, montmorillonite K10, and I2, have been used to catalyze the reaction. Most of such methods developed up to the middle of 2009 have been enumerated in a recent elaborate review done by Shiri et al. Citation12. However, in spite of their potential utility, some of the reported methods suffer from drawbacks such as long reaction time, expensive catalyst, low yield, and cumbersome product isolation procedures. In order to alleviate such drawbacks, development of newer catalytic conditions has become necessary. It is worthwhile to point out here that even after publication of the above-mentioned review, a sizable number of methods have been reported in the literature through use of newer catalysts, mainly with a view to decreasing the reaction time and making the process more and more simple and eco-friendly. Thus, mention may be made of at least 12 very recent methods, seven of which were performed under solvent-free condition using catalysts such as alum [KAl(SO4)2·12H2O] Citation13, LaCl3·7H2O Citation14, boric acid Citation15, 3-methyl-1-sulfonic acid imidazolium chloride Citation16, combination of N,N,N,N-tetrabromobenzene-1,3-disulfonamide (TBBDA) and poly(N-bromobenzene-1,3-disulfonamide (PBBS) Citation17, silicotungstic acid Citation18, and SnCl2·2H2O Citation19 and the others done by use of the catalysts such as SBA-15-supported PSFSI (Solvent: DCM) Citation20, phosphorodiamidic acid (toluene) Citation21, and Fe(HSO4)3 (DCM) Citation22, silica-supported cupric fluoroborate [Cu(BF4)2·SiO2] (DCM) Citation23 and glycerol as a promoting medium Citation24.
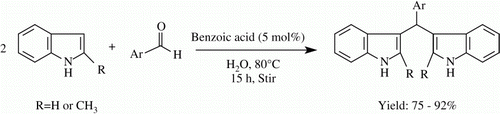
The use of water as a reaction medium is considered to be cheap, safe, and environmentally benign. Hydrophobic effect Citation25 shown by many organic substrates and internal pressure of water (and its increase by added electrolytes) are two very important factors which enhance the rates of organic reactions involving decrease in the number of molecules. Again, development of chemical processes without use of any catalyst has currently received considerable importance in synthetic organic chemistry. Recently, Cozzi and Zoli Citation26 have demonstrated the direct substitution of optically active ferrocenyl alcohols “on-water” with indole, pyrrole, and thiophenols, which gives the product in good yield without the use of Lewis acids, Bronsted acids, or surfactants. In another paper, the same authors Citation27 have reported that not only ferrocenyl alcohols but also a variety of other alcohols undergo above type of direct nucleophilic substitution “on-water” without the use of Lewis acids, Bronsted acids, or surfactants. Regarding synthesis of BIMs in water, Kamal and Qureshi Citation28 reported first that these compounds could be obtained by using acetic acid at pH 2.5 at room temperature for over 10 days. They pointed out that no condensation took place in water alone. Subsequently, several other methods for reaction in water have been reported Citation29, Citation30. We report herein the direct reaction of indole with aromatic aldehydes “on-water” without the use of any catalyst (five examples) and also using ca. 5 mol% benzoic acid, a cheap and very common desk reagent, as catalyst (12 examples).
Results and discussion
A suspension of a mixture of indole (1a) and benzaldehyde (2a) (mole ratio 1:2) in water was subjected to heating at 80°C with vigorous stirring until the starting materials disappeared (ca. 15 h). Product isolation from the resulting emulsion-like material gave a pure compound (vide Experimental), characterization of which from analytical and spectral data showed it to be bis(3-indolyl)phenylmethane (3a) (). After getting success in the reaction between benzaldehyde and indole done in the above way, we studied the reactions of indole with 11 other aromatic aldehydes, viz., p-methylbenzaldehyde (2b), furfural (2c), p-methoxybenzaldehyde (2d), 3,4-dimethoxybenzaldehyde (2e), piperonal (2f), p-chlorobenzaldehyde (2g), p-bromobenzaldehyde (2h), p-nitrobenzaldehyde (2i), m-nitrobenzaldehyde (2j), vanillin (2k) and 4-N,N-dimethylaminobenzaldehyde (2l), and chromone-3-aldehyde (2m) under similar reaction conditions. It was interesting to note that among the above-mentioned aromatic aldehydes only 2b and 2c were found to undergo reaction to a very significant extent to give BIMs in very good yield. A careful analysis of this observation led to the suggestion that only those aldehydes that are very susceptible to aerial oxidation gave BIMs in substantial yield. This may be accounted for by considering that during heating of the reaction mixtures in air, these aldehydes are oxidized to yield corresponding carboxylic acids which in low concentration act as catalysts for the BIM formation. This contention was proved to be correct when the extent of BIM formation was found to increase very significantly from any one of the aldehydes 2d–m under the same reaction condition by use of a small amount of benzoic acid as catalyst. 2-Methylindole was treated in the same way and here also the corresponding BIMs were obtained in very good yield. All the results are presented in .
Table 1. Synthesis of bis(3-indolyl)methanes in water.
Experimental
All products () were characterized from their spectral data and their physical properties were compared with those reported in the literature.
General procedure for synthesis of bis (3-indolyl) methanes
A mixture of an aromatic aldehyde (1 mmol) and indole (2 mmol) was taken in 50 ml distilled water in a round-bottomed flask. Initially the reactants floated on the water surface owing to their low solubility. The mixture was then subjected to heating at 80°C with vigorous stirring for 15 h. It was then cooled and saturated sodium bicarbonate solution was added dropwise till effervescence ceased. Extraction with chloroform followed by chromatography of the concentrate of the extract over silica gel using petroleum ether-ethyl acetate (9:1) as eluent afforded pure BIM [3a–c in very good yield and others in poor yield]. To increase the yield of BIMs from the aldehydes 2d–m, the same reaction was repeated by addition of a small amount of benzoic acid (5 mol%). Work up of the reaction mixture in the same way gave pure BIMs (3d–m) in good to excellent yield. Reactions with 2-methylindole were also performed in the same way. Most of the BIMs Citation3 were known compounds (referred in ). All the synthesized BIMs were characterized from their physical, analytical, and spectral data. The spectral data of some selected compounds are given in the following section.
Spectral data for selected compounds
Bis(3-indolyl)-(4-methylphenyl)methane (3b)
Light pink crystalline solid, IR (KBr, cm−1): 3408 (N–H), 2950, 1606, 1510, 1217, 772; 1H NMR (300 MHz, CDCl3): δ 2.37 (s, 3H, Ar–CH3), 5.85 (s, 1H, Ar–CH<), 6.67 (s, 2H), 7.00 (t, 2H, J=7.2 Hz), 7.08 (d, 2H, J=7.2 Hz), 7.17 (t, 2H, J=7.5 Hz), 7.23 (d, 2H, J=8.1 Hz), 7.35 (d, 2H, J=8.1 Hz), 7.39 (d, 2H, J=7.9 Hz), 7.91 (br s, 2H, NH); Anal. Calcd. for C24H20N2 C, 85.68; H, 5.99; N, 8.33%; found: C, 85.49; H, 6.26; N, 8.04%.
Bis(3-indolyl)-(3,4-dimethoxyphenyl)methane (3e)
Pinkish crystalline solid; IR (KBr, cm−1): 3444 (N–H), 3050, 2990, 1623, 1493, 1217, 1050, 760; 1H NMR (200 MHz, CDCl3): δ 3.76 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 5.83 (s, 1H, Ar–CH < ), 6.67 (s, 2H), 6.76 (d, 1H, J=8.4 Hz), 6.84 (d, 1H, J=8.2 Hz), 6.92 (s, 1H), 7.00 (t, 2H, J=7.6 Hz), 7.17 (t, 2H, J=8.0 Hz,), 7.38 (t, 4H, J=8.0 Hz,), 7.93 (br s, 2H, NH); 13C NMR (75 MHz, CDCl3): δ = 148.62, 147.23, 136.64, 127.01, 125.99, 124.18, 123.45, 121.85, 120.49, 119.89, 119.15, 112.13, 110.94, 110.81, 55.76 (OCH3), 55.73 (OCH3), 39.73 (Ar–CH<); Anal. Calcd. for C25H22N2O2 C, 78.51; H, 5.80; N, 7.32%; found: C, 78.36; H, 5.91; N, 7.20%.
Bis(3-indolyl)-(3,4-methylenedioxyphenyl)methane (3f)
Pinkish crystalline solid; IR (KBr, cm−1): 3412 (N–H), 2950, 1610, 1510, 1216, 750; 1H NMR (300 MHz, CDCl3): δ = 5.81 (s, 1H, Ar–CH<), 5.90 (s, 2H, –OCH2O–), 6.69 (br. s, 2H), 6.72 (d, 1H, J=8.1 Hz,), 6.82 (br. s, 1H), 6.86 (d, 1H, J=8.1 Hz,), 7.01 (t, 2H, J=7.2 Hz), 7.17 (t, 2H, J=7.8 Hz), 7.55 (d, 2H, J=8.1Hz), 7.40 (d, 2H, J=7.8 Hz), 7.93 (br. s, 2H, NH); Anal. Calcd. for C24H18N2O2: C, 78.67; H, 4.95; N, 7.65%; found C, 78.45; H, 5.21; N, 7.58%.
Bis(3-indolyl)-(4-nitrophenyl)methane (3i)
Yellow crystalline solid; IR (KBr, cm−1): 3418 (N–H), 3041, 1599, 1350, 1225, 771; 1H NMR (300 MHz, CDCl3): δ = 5.99 (s, 1H, Ar–CH<), 6.69 (br. s, 2H), 7.02 (t, 2H, J=7.2 Hz), 7.20 (t, 2H, J=7.2 Hz,), 7.33 (d, 2H, J=8.1 Hz), 7.39 (d, 2H, J=8.1 Hz), 7.51 (d, 2H, J=8.1 Hz), 8.02 (br. s, 2H, NH), 8.14 (d, 2H, J=8.1 Hz); 13C NMR (75 MHz, CDCl3): δ = 151.83, 146.57, 136.69, 129.52, 126.65, 123.63, 122.36, 119.61, 119.56, 118.14, 111.25, 40.21 (Ar–CH<); Anal. Calcd. for C23H17N3O2: C, 75.19; H, 4.66; N, 11.44%; found C, 75.02; H, 4.75; N, 11.30%.
Bis(3-indolyl)-(4-N,N-dimethylaminophenyl)methane (3l)
Pinkish crystalline solid; IR (KBr, cm−1): 3470 (N–H), 3051, 1520, 1462, 1350, 1226, 761; 1H NMR (200 MHz, CDCl3): δ = 2.91 (s, 6H, NMe2), 5.80 (s, 1H, Ar–CH<), 6.66–6.69 (m, 4H), 6.98 (t, 2H, J=7.5 Hz), 7.12–7.21 (m, 4H), 7.34 (d, 2H, J=8.1 Hz,) 7.41 (d, 2H, J=8.1 Hz), 7.88 (br. s, 2H, NH); Anal. Calcd. for C25H23N3, C, 82.16; H, 6.34; N, 11.50%, found C, 81.98; H, 6.59; N, 11.32%.
Bis(3-indolyl)-(chromone-3-yl)methane (3m)
Cream-colored crystalline solid; IR (KBr, cm−1): 3336, (N–H), 1628 (C = O), 1616, 1599, 1466, 1216, 850, 736; 1H NMR (200 MHz, CDCl3): δ = 6.25 (s, 1H, 3-Chr.-CH < ), 6.91 (s, 2H), 7.05 (t, 2H, J=7.2 Hz), 7.19 (t, 2H, J=7.2 Hz), 7.35–7.43 (m, 4H), 7.52 (d, 2H, J=7.6 Hz), 7.65 (br. t, 1H, J=7.2 Hz), 7.69 (s, 1H, H-2 of Chr.), 8.01 (br. s, 2H, NH), 8.27 (br. d, 1H, H-5 of Chr.).
Bis(2-methyl-3-indolyl)-(3,4-methylenedioxyphenyl)methane (3p)
Pinkish crystalline solid; IR (KBr, cm-1): 3384 (N–H), 3049, 1608, 1500, 1485, 1229, 1040, 937, 756; 1H NMR (200 MHz, CDCl3): δ = 2.06 (s, 6H, 2×CH3), 5.91 (s, 3H, –O–CH2–O– and Ar–CH < ), 6.67–7.26 (m, 11H, Ar–H), 7.73 (br. s, 2H, NH); Anal. Calcd. for C26H22N2O2, C, 79.16; H, 5.62; N, 7.10%, found C, 78.87; H, 5.75; N, 6.86%.
Bis(2-methyl-3-indolyl)-(4-chlorophenyl)methane (3q)
Pinkish crystalline solid; IR (KBr, cm−1): 3381 (N–H), 3047, 1602, 1487, 1428, 1304, 1243, 1086, 1013, 822, 745; 1H NMR (200 MHz, CDCl3): δ = 2.08 (s, 6H, 2×CH3), 5.97 (s, 1H, Ar–CH < ), 6.88 (t, 2H, J=7.2 Hz), 6.98 (d, 2H, J=7.6 Hz), 7.06 (t, 2H, J=7.8 Hz), 7.21 (s, 4H, 4–Cl–C6H4–), 7.26 (d, 2H, J=7.5 Hz), 7.77 (br. s, 2H, NH); Anal. Calcd. for C25H21N2Cl, C, 78.01; H, 5.50; N, 7.28%, found C, 77.79; H, 5.39; N, 7.02%.
Conclusions
We have described an electrophilic substitution reaction of indole “on-water” for generation of BIMs without the use of any Lewis acid catalyst or surfactant, where aerial oxidation of the aldehyde used is very significant. In other cases, addition of a trace amount of benzoic acid (5 mol%) is required. The mild reaction condition, the use of water without the presence of co-solvent, utilization of a small amount of an inexpensive catalyst, and very good yield of the products are all plus points of this methodology. Moreover, it is environmentally benign.
Acknowledgements
Financial assistance from the UGC-CAS and DST-PURSE programs, Department of Chemistry, Jadavpur University, is gratefully acknowledged. The authors also thank Prof. D. Mal, Department of Chemistry, IIT, Khargarpur, and the DST-FIST program to the Department of Chemistry, Jadavpur University for providing the NMR spectral data. CG is thankful to UGC, New Delhi, for a Research Fellowship.
References
- Murakatake , H. ; Kumagami , H. ; Natsume , M. Tetrahedron 1990 , 46 , 6351 – 6360 .
- Vailancouirt , V. ; Albizati , K.F. J. Am. Chem. Soc . 1993 , 115 , 3499 – 3502 .
- Fukuyama , T. ; Chen , X. J. Am. Chem Soc . 1994 , 116 , 3125 – 3126 .
- Kobayashi , M. ; Aoki , S. ; Gato , K. ; Matsunami , K. ; Kurosu , M. ; Kitagawa , I. Chem. Pharm. Bull . 1994 , 42 , 2449 – 2451 .
- Bell , R. ; Carmeli , S. ; Sar , N. J. Nat. Prod . 1994 , 57 , 1587 – 1590 .
- Sujatha , K. ; Perumal , P.T. ; Muralidharan , D. ; Rajendra , M. Indian J. Chem . 2009 , 48B , 267 – 272 .
- Giannini , G. ; Marzi , M. ; Tiuti , M.O. ; Pisano , C. Chem. Abstr . 2000 ; 136 , 355230 .
- Safe , S. ; Papineni , S. ; Chintharlapalli , S. Cancer Lett . 2008 , 269 , 326 – 338 .
- Inamoto , T. ; Papineni , S. ; Chintharlapalli , S. ; Cho , S.D. ; Safe , S. ; Kamat , A.M. Mol. Cancer Ther . 2008 , 7 , 3825 – 3833 .
- McDougal , A. ; Gupta , M.S. ; Morrow , D. ; Ramamoorthy , K. ; Lee , J.E. ; Safe , S.H. Breast Cancer Res. Treat . 2001 , 66 , 147 – 157 .
- Wang , T.T.Y. ; Milner , M.J. ; Milner , J.A. ; Kim , Y.S. J. Nutr. Biochem . 2006 , 17 , 659 – 664 .
- Shiri , M. ; Zolfigol , M.A. ; Kruger , H.G. ; Tanbakouchian , Z. Chem. Rev . 2010 , 110 , 2250 – 2293 .
- Sonar , S.S. ; Sadaphal , S.A. ; Kategaonkar , A.H. ; Pokalwar , R.U. ; Shingate , B.B. ; Shingare , M.S. Bull. Korean Chem. Soc . 2009 , 30 , 825 – 828 .
- Seyedi , N. ; Saidi , K. ; Khabazzadeh , H. Synth. Commun . 2009 , 39 , 1864 – 1870 .
- Jadav , J.S. ; Gupta , M.K. ; Jain , R ; Jadav , N.N ; Reddy , B.V.S. Monatsh. Chem . 2010 , 141 , 1001 – 1004 .
- Zolfigol , M.A. ; Khazaei , A. ; Moosavi-Zare , A.R. ; Zare A. Org. Prep. Proced. Int . 2010 , 42 , 95 – 102 .
- Ghorbani-Vaghei , R ; Malaekehpoor , S.M. Org. Prep. Proced. Int . 2010 , 42 , 175 – 182 .
- Li , J.-T. ; Sun , S.-F. E-J. Chem . 2010 , 7 , 922 – 926 .
- Shaikh , K.A. ; Mohammed , Z.A. ; Patel , N.T. ; Syed , S.A. ; Patil , V.A. Res. J. Pharm. Biol. Chem. Sci . 2010 , 1 , 730 – 736 .
- Ma , Z.-H. ; Han , H.-B. ; Zhou , Z.-B. ; Nie , J. J. Mol. Cat. A: Chem . 2009 , 311 , 46 – 53 .
- He , Q.-L. ; Sun , F.-L. ; Zheng , X.-J. ; You , S.-L. Synlett 2009 , 1111 – 1114 .
- Rahimizadeh , M. ; Eshghi , H. ; Bakhtiarpoor , Z. ; Pordel , M J. Chem. Res . 2009 , 269 – 270 .
- Meshram , G.A. ; Patil , V.S. Synth. Commun . 2010 , 40 , 29 – 38 .
- He , F. ; Li , P. ; Gu , Y. ; Li , G. Green Chem . 2009 , 11 , 1767 – 1773 .
- Li , C.-J. ; Chan , T.H. Organic Reactions in Aqueous Media ; John Wiley & sons Inc , New York , 1997 , 1 – 12 .
- Cozzi , P.G. ; Zoli , L. Angew. Chem. Int. Ed . 2008 , 47 , 4162 – 4166 .
- Cozzi , P.G. ; Zoli , L. Green Chem . 2007 , 9 , 1292 – 1295 .
- Kamal , A. Qureshi , A.A. Tetrahedron 1963 , 19 , 513 – 520 .
- Austin , J.E. ; Fraenkel-Conrat , H. Proc. Nat. Acad. Sci. U.S.A . 1992 , 89 , 8439 – 8442 .
- Chen , D. ; Yu , L. ; Wang , P.G. Tetrahedron Lett . 1996 , 37 , 4467 – 4470 .
- Yadav , J.S. ; Reddy , B.V.S. ; Murthy , C.V.S.R. ; Kumar , G.M. ; Madan , C. Synthesis 2001 , 783 – 787 .
- Ji , S.-J. ; Wang , S.-Y ; Zhang , Y. ; Loh , T.-P. Tetrahedron 2004 , 60 , 2051 – 2055 .
- Mallik , A.K. ; Pal , R. ; Mandal , T.K. Indian J. Chem . 2007 , 46B , 2056 – 2059 .
- Sosnovskikh , V.Y. ; Irgashev , R.A. ; Levchenko , A.A. Tetrahedron 2008 , 64 , 6607 – 6614 .
- Deb , M.L. ; Bhuyan , P.J. Tetrahedron Lett . 2006 , 47 , 1441 – 1443 .