Abstract
We present here a clean and fast synthesis of organic disulfides starting from thiols using glycerol as solvent under microwave irradiation. This efficient method is general for aromatic, aliphatic, and functionalized thiols, affording the corresponding disulfides in good to excellent yields after easy work up. Glycerol can be easily recovered and utilized for further oxidation reactions.
1. Introduction
In organic synthesis, the choice of the solvent is a crucial step in a chemical reaction. The development of green solvents from renewable resources has gained much interest recently because of the extensive uses of solvents in almost all of the chemical industries and of the predicted disappearance of fossil oil Citation1–6. The wanted characteristics for a green solvent include no flammability, high availability, obtaining from renewable sources, and biodegradability Citation6. With the increase in biodiesel production worldwide, the market saturation of glycerol, a side product of biodiesel production, is inevitable Citation7. The peculiar physical and chemical properties, such as polarity, low toxicity, biodegradability, high boiling point, and ready availability from renewable feed stocks Citation8 prompted recently the use of glycerol Citation9–12 and their eutectics Citation13 as a green solvent in organic synthesis. Heck and Suzuki cross-couplings, ring closing metathesis of diolefins, multicomponent reactions, base- and acid-promoted condensations, catalytic hydrogenation, asymmetrical reduction, and cycloisomerization of (Z)-enynols into furans are some examples of the use of glycerol as a solvent in organic reactions Citation9–12.
On the other hand, thiols and disulfides are important in both biological Citation14–19 and chemical process Citation20–22 . Disulfides are useful reagents in organic synthesis Citation20–22 and essential moieties of biologically active compounds for peptide and protein stabilization Citation14–19. As disulfides are relatively more stable to organic reactions such as oxidation, alkylation, and acylation compared to the corresponding free thiols, the thiol group can conveniently be protected as a disulfide. Besides, there are a large number of commercially available thiols and the interconversion between thiols and disulfides is easy Citation23. These aspects are responsible for the continuous interest in development of new, selective and efficient protocols for the preparation of disulfides Citation20–22, Citation24–34. Recently reported procedures involve the use of stoichiometric amount of anhydrous potassium phosphate Citation24, potassium permanganate Citation25, molecular bromine supported on silica gel Citation26, N-phenyltriazolinedione Citation27, VO(acac)2 Citation28, trichloroisocyanuric acid Citation29, nitric acid Citation30, 1,3-dibromo-5,5-dimethylhydantoin Citation31, basic alumina Citation32, CsF-Celite Citation33, and montmorillonite K10 Citation34.
The development of environmentally benign and clean synthetic methods for the synthesis of disulfides, including those involving solvent-free or the use of alternative solvents, such as water and ionic liquids, has increased in recent years Citation27, Citation35–44. These methods involve the use of N-phenyltriazolinedione Citation27, pyridinium chlorochromate Citation35, 1,3-dibromo-5,5-dimethylhydantoin Citation36, SO2Cl2 Citation37, trichloronitromethane Citation38, KMnO4/MnO2 Citation39, KMnO4 supported on montmorillonite K10 Citation40, catalytic amount of iodine and CeCl3·7H2O in graphite Citation41, KF/Al2O3 Citation42, [bmim][SeO2(OCH3)] Citation43, and [bmim][BF4] Citation44.
Despite several advantages, the solvent-free methods are restricted to systems where at least one of the reagents is liquid at room temperature, whereas the uses of ionic liquids, especially imidazolium systems with PF6 and BF4 anions, have some drawbacks, such as the high cost and liberation of hazardous HF during recycling Citation45–48. Thus, the use of alternative nonvolatile green solvents, such as glycerol, has been shown as an attractive way to cleaner synthesis of disulfides. According to our interest in the green protocols in organic chemistry (42, 43, Citation49–55, we describe here the use of glycerol as a green solvent in the oxidation of thiols to disulfides ().
2. Results and discussion
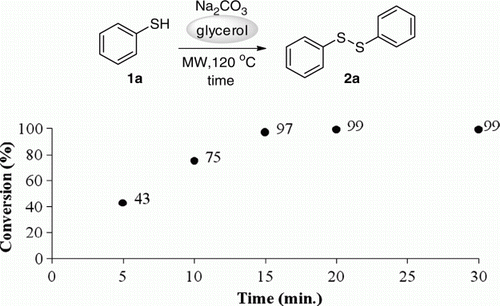
Firstly, we reacted benzenethiol 1a with K2CO3 as base, using glycerol as solvent at room temperature and under these conditions, no product was observed. When the reaction was performed at room temperature under microwave irradiation, product 2a was formed in 15% yield after 30 minutes. Encouraged for this result, we performed this oxidation reaction in microwave irradiation under different temperatures (). To our satisfaction, increasing the temperature to 120°C the reaction proceeds smoothly, furnishing the disulfide 2a in excellent yield (; entry 5) and the same result was obtained at 150°C (; entry 6).
Table 1. Optimization of oxidation reaction.a
We observed that the nature of the base was critical for the success of the oxidation. When the reaction was carried out with different bases such as: Na2CO3, Cs2CO3, Na3PO4, K3PO4, KOH, LiOAc, NaOAc, and Et3N, benzenethiol 1a was successfully oxidated (; entries 7–14), and the best results were obtained using Na2CO3 and Cs2CO3 as base. Gratifyingly, the use of Na2CO3, an inexpensive base, resulted in the oxidation of benzenethiol 1a in 92% isolated yield (; entry 7). It is also important to mention that when the reaction was performed without base no product was obtained (; entry 15). Reaction of benzenethiol 1a with Na2CO3 in glycerol was performed using a conventional heating and the product 2a was isolated in a good yield, however, in a longer reaction time comparing with microwave-assisted method (; entry 7 vs. 16).
To obtain an efficient methodology in terms of energy economy, we realized a study to establish the minimum time associated with a good reaction rate under the optimized conditions (). Analyzing , excellent conversion of benzenethiol in diphenyl disulfide 2a was observed after 15 minutes of reaction. A further decrease in the reaction time (less than 15 minutes) was followed by a considerable reduction in the conversion rate of benzenethiol.
In an optimized reaction, benzenethiol 1a (1.0 mmol) and Na2CO3 (1.1 mmol) were dissolved in glycerol (1.0 mL) and reacted under microwave irradiation at 120°C for 15 minutes, yielding 2a in 92% isolated yield.
After reaction optimization, a study regarding the recovering and reusing of glycerol was performed. After the total consumption of benzenethiol 1a, the reaction mixture was diluted and extracted with a mixture of hexane/ethyl acetate 95:5 (3×3 mL). The upper phase was dried and the solvent evaporated. The inferior, glycerol phase was dried under vacuum and directly reused. We observed that the glycerol phase reused furnishing diphenyl disulfide 2a in 89%. However, after second reuse, only traces of diphenyl disulfide 2a were obtained. In view of this result, we performed the reuse studies using NaCO3 in all runs, as shown in . It was observed that a good level of efficiency was maintained even after being reused four times. Diphenyl disulfide 2a was obtained in 92%, 92%, 90%, 89%, and 88% yields after successive cycles, however, with an increase in the reaction time after the third run.
Table 2. Reuse of glycerol.a
To demonstrate the generality of this method, we prepared a series of organic disulfides 2a–t using aryl, heteroaryl or alkyl thiols, Na2CO3, and glycerol under microwave irradiation (). In most cases, the reactions proceeded fast and smoothly to give disulfides 2a–t in good to excellent yields. A structurally diverse range of aryl thiols were oxidated to corresponding diaryl disulfides in excellent yields (; entries 1–12). Diaryl disulfides containing electron donating (EDG) (; entries 2–6) and electron withdrawing groups (EWG) (; entries 7–10) could be obtained in high yields. Both aryl thiols containing EDG and EWG have no significant influence on the reactivity of the process, because the products were obtained in comparable yields. Satisfactory yields of oxidation were achieved using 2-amino-4-chlorobenzenethiol, 2-naphthalenethiol, and 2-mercaptobenzothiazole (; entries 11–13). Good results were obtained using benzylic or furfuryl thiols yielding the corresponding disufides 2n–p in 84–87%, but in 30 minutes reaction time (, entries 14–16). Finally, when we employed alkylic thiols, the corresponding dialkyl disulfides 2q–t were synthesized in good yields (; entries 17–20).
Table 3. Scope and generality of the synthesis of disulfides 2a–t using glycerol as solvent.a
3. Experimental section
3.1. General remarks
Proton nuclear magnetic resonance spectra (1H NMR) were obtained at 200 MHz on a Bruker DPX-200 NMR spectrometer. Spectra were recorded in CDCl3 solutions. Chemical shifts are reported in parts per million, referenced to the solvent peak of CDCl3 or tetramethylsilane (TMS) as the external reference. Data are reported as follows: chemical shift (δ), multiplicity, coupling constant (J) in Hertz, and integrated intensity. Carbon-13 nuclear magnetic resonance spectra (13C NMR) were obtained at 50 MHz on a Bruker DPX-200 NMR spectrometer. Spectra were recorded in CDCl3 solutions. Chemical shifts are reported in parts per million, referenced to the solvent peak of CDCl3. Column chromatography was performed using Merck Silica Gel (230–400 mesh) following the standard methods. Thin layer chromatography (TLC) was performed using Merck Silica Gel GF254, 0.25 mm thickness. For visualization, TLC plates were placed under ultraviolet light, stained with iodine vapor, or acidic vanillin. The reactions were monitored by TLC for disappearance of starting material. All microwave reactions were conducted using a CEM Discover, mode operating systems working at 2.45 GHz, with a power programmable from 1 to 300 W.
3.2. General procedure for the oxidation of thiols using glycerol
In a 10 mL glass vial equipped with a small magnetic stirring bar, containing Na2CO3 (0.116 g; 1.1 mmol) and glycerol (1 mL), thiol (1.0 mmol) was added. The vial was tightly sealed with an aluminum/Teflon crimp top. The mixture was then irradiated in a microwave reactor (CEM Explorer) for 15 minutes at 120°C (temperature was measured with an IR sensor on the outer surface of the reaction vial), using an irradiation power of 100 W and pressure of 100 psi (the ramp temperature rate was 45 sec). After the reaction was complete, the product was extracted by successive washings with a mixture of hexane/ethyl acetate (95:5) (3×3 mL) and concentrated under vacuum. The residue was purified by column chromatography on silica gel using hexane/ethyl acetate as the eluent.
1,2-diphenyl disulfide 2a Citation56. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.41–7.39 (m, 4H); 7.22–7.07 (m, 6H). 13C NMR (50 MHz, CDCl3): δ (ppm) 136.33, 129.10, 127.50, 127.30.
1,2-bis(2-tolyl) disulfide 2b Citation57. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.52–7.49 (m, 2H); 7.15–7.10 (m, 6H); 2.42 (s, 6H). 13C NMR (50 MHz, CDCl3): δ (ppm) 137.42, 135.40, 130.31, 127.78, 127.29, 126.75, 21.08.
1,2-bis(3-tolyl) disulfide 2c Citation58. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.34–7.21 (m, 8H); 2.13 (s, 6H). 13C NMR (50 MHz, CDCl3): δ (ppm) 139.92, 137.19, 130.52, 129.89, 128.22, 127.75, 21.82.
1,2-bis(4-tolyl) disulfide 2d Citation59. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.40 (d, J=8.3 Hz, 4H); 7.12 (d, J=8.3 Hz, 4H); 2.33 (s, 6H). 13C NMR (50 MHz, CDCl3): δ (ppm) 139.02, 131.89, 130.78, 128.91, 21.01.
1,2-bis(4-methoxyphenyl) disulfide 2e Citation60. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.40 (d, J=8.1 Hz, 4H); 6.84 (d, J=8.1 Hz, 4H); 3.80 (s, 6H). 13C NMR (50 MHz, CDCl3): δ (ppm) 159.91, 132.67, 128.42, 114.61, 55.36.
bis(4-aminophenyl) disulfide 2f Citation61. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.20 (d, J=8.5 Hz, 4H); 6.53 (d, J=8.5 Hz, 4H); 3.73 (bs, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 144.75, 130.55, 127.95, 118.60.
1,2-bis(2-chlorophenyl) disulfide 2g Citation59. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.55 (dd, J 1 =7.6 Hz, J 2 =1.5 Hz, 2H); 7.35 (dd, J 1 =7.6 Hz, J 2 =1.5 Hz, 2H); 7.23–7.11 (m, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 134.33, 132.37, 131.32, 129.29, 128.98, 124.75.
1,2-bis(4-chlorophenyl) disulfide 2h Citation62. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.40 (d, J=7.8 Hz, 4H); 7.13 (d, J=7.8 Hz, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 135.07, 132.19, 130.43, 128.74.
1,2-bis(4-bromophenyl) disulfide 2i Citation63.1H NMR (200 MHz, CDCl3): δ (ppm) 7.42 (d, J=8.2 Hz, 4H), 7.33 (d, J=7.8 Hz, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 133.03, 131.31, 130.24, 128.61.
1,2-bis(4-fluorophenyl) disulfide 2j Citation64. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.40 (m, 4H); 6.95 (t, J=8.6 Hz, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 163.28 (d, J=246.5 Hz), 133.84 (d, J=2.9 Hz) 132.78 (d, J=8.6 Hz), 118.00 (d, J=21.1 Hz).
6,6′-disulfanediyl bis(3-chloroaniline) 2k Citation65. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.15–6.76 (m, 6H); 5.60 (bs, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 144.98, 132.52, 128.06, 123.86, 123.36, 123.23.
2-Naphthyl disulfide 2l Citation66. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.99 (d, J=0.9 Hz, 2H), 7.81–7.72 (m, 4H), 7.65–7.61 (m, 2H), 7.49–7.42 (m, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 134.03, 133.51, 132.55, 128.91, 127.84, 127.54, 126.73, 126.60, 126.21, 125.70.
1,2-bis(2-benzothiazolyl) disulfide 2m Citation67. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.37 (dd, J 1 =3.5 Hz, J 2 =0.9 Hz, 2H); 7.32–7.25 (m, 4H); 7.25–7.21 (m, 2H). 13C NMR (50 MHz, CDCl3): δ (ppm) 149.26, 145.86, 133.61, 127.24, 125.14, 120.86, 120.69.
1,2-dibenzyl disulfide 2n Citation59. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.36–7.22 (m, 10H); 3.60 (s, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 137.20, 129.20, 128.20, 127.20, 43.32.
1,2-bis(4-chlorobenzyl) disulfide 2o Citation68. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.29–7.27 (m, 4H); 7.15–7.13 (m, 4H); 3.57 (s, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 134.60, 133.02, 130.11, 127.96, 43.16.
1,2-difurfuryl disulfide 2p Citation69. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.40–7.38 (m, 2H); 6.34–6.32 (m, 2H); 6.22 (d, J=3.2 Hz, 2H); 3.69 (s, 4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 149.46, 142.01, 111.85, 107.46, 35.99.
1,2-di(n-propyl) disulfide 2q Citation70. 1H NMR (200 MHz, CDCl3): δ (ppm) 2.41 (t, J=7.2 Hz, 4H); 1.46 (quint, J=7.2, 4H); 0.88 (t, J=7.2 Hz, 6H). 13C NMR (50 MHz, CDCl3): δ (ppm) 41.26, 22.56, 13.12.
1,2-di(n-octyl) disulfide 2r Citation71. 1H NMR (200 MHz, CDCl3): δ (ppm)): 2.65 (t, J=8.0 Hz, 14H); 1.77–1.18 (m, 24H); 0.87 (t, J=7.2 Hz, 6H). 13C NMR (50 MHz, CDCl3): δ (ppm) 39.84, 32.14, 29.72, 29.38, 28.63, 28.28, 22.72, 14.10.
1,2-di(t-butyl) disulfide 2s Citation63. 1H NMR (200 MHz, CDCl3): δ (ppm) 1.29 (s, 18H). 13C NMR (50 MHz, CDCl3): δ (ppm) 45.71, 30.51.
1,2-bis-hydroxymethyl disulfide 2t Citation72. 1H NMR (200 MHz, CDCl3): δ (ppm) 4.64 (s, 2H), 4.32 (4H). 13C NMR (50 MHz, CDCl3): δ (ppm) 64.81.
3.3. General procedure for the recycle of glycerol
In a 10 mL glass vial equipped with a small magnetic stirring bar, containing Na2CO3 (0.116 g; 1.1 mmol) and glycerol (1 mL), thiol (1.0 mmol) was added. The vial was tightly sealed with an aluminum/Teflon crimp top. The mixture was then irradiated in a microwave reactor (CEM Explorer, mode operating systems working at 2.45 GHz) for 15 minutes at 120°C (temperature was measured with an IR sensor on the outer surface of the reaction vial), using an irradiation power of 100 W and pressure of 100 psi. After the reaction was complete, the product was extracted by successive washings with a mixture of hexane/ethyl acetate (95:5) (3×3 mL) and concentrated under vacuum. The residue was purified by column chromatography on silica gel using hexane/ethyl acetate as the eluent.
4. Conclusion
In conclusion, glycerol has proved to be an efficient solvent for the oxidation of aromatic, aliphatic, and functionalized thiols under microwave irradiation. The reactions proceeds quickly, and the desired disulfides were obtained in good to excellent yields. In addition, glycerol can be easily recovered and utilized for further oxidation reactions and is particularly appropriate to the green chemistry concept.
Acknowledgements
The authors are grateful to FAPERGS (FAPERGS/PRONEX 10/0005-1 and 10/0027-4), CAPES, FINEP, and CNPq for the financial support.
References
- Handy , S.T. Chem. Eur. J . 2003 , 9 , 2938 .
- Leitner , W. Green Chem . 2007 , 9 , 923 .
- Horváth , I.T. Green Chem . 2008 , 10 , 1024 .
- Giovanni , I. ; Silke , H. ; Dieter , L. ; Burkhard , K. Green Chem . 2006 , 8 , 1051 .
- Clark , J.H. Green Chem . 1999 , 1 , 1 .
- Nelson , W.M. Green Solvents for Chemistry: Perspectives and Practice ; Oxford : Oxford University Press , 2003 .
- Johnson , D.T. ; Taconi , K.A. Environ. Prog . 2007 , 26 , 338 .
- Pagliaro , M. ; Rossi , M. In The Future of Glycerol: New Usages for a Versatile Raw Material ; Clark , J.H. , Kraus , G.A. ; Cambridge : RSC Green Chemistry Series , 2008 .
- Gu , Y. ; Jèrôme , F. Green Chem . 2010 , 12 , 1127 .
- Bakhrou , N. ; Lamaty , F. ; Martinez , J. ; Colacino , E. Tetrahedron Lett . 2010 , 51 , 3935 .
- Li , M. ; Chen , C. ; He , F. ; Gu , Y. Adv. Synth. Catal . 2010 , 352 , 519 .
- Francos , J. ; Cadierno , V. Green Chem . 2010 , 12 , 1552 .
- Abbott , A.P. ; Harris , R.C. ; Ryder , K.S. ; D'Agostino , C. ; Gladden , L.F. ; Mantle , M.D Green Chem . 2011 , 13 , 82 .
- Bodanszky , M. Principles of Peptide Synthesis Springer-Verlag Berlin 1984 ; 307 .
- Jocelyn , P.C. Biochemistry of the Thiol Group ; New York : American Press , 1992 .
- Kanda , Y. ; Fukuyama , T. J. Am. Chem. Soc . 1993 , 115 , 8451 .
- Palmer , B.D. ; Newcastle , G.W. ; Thompson , A.M. ; Boyd , M. ; Showalter , H.D.H. ; Sercel , A.D. ; Fry , D.W. ; Kraker , A.J. ; Dennyyrosine , W.A. J. Med. Chem . 1995 , 38 , 58 .
- Schmidt , B. ; Lindman , S. ; Tong , W. ; Lindeberg , G. ; Gogoll , A. ; Lai , Z. ; Thornwall , M. ; Synnergren , B. ; Nilson , A. ; Welch , C.J. ; Sohtell , M. ; Westerlund , C. ; Nyberg , F. ; Karlen , A. ; Hallberg , A. J. Med. Chem . 1997 , 40 , 903 .
- Lyukmanova , E.N. ; Shulepko , M.A. ; Tikhonov , R.V. ; Shenkarev , Z.O. ; Paramonov , A.S. ; Wulfson , A.N. ; Kasheverov , I.E. ; Ustich , T.L. ; Utkin , Y.N. ; Arseniev , A.S. ; Tsetlin , V.I. ; Dolgikh , D.A. ; Kirpichnikov , M.P. Biochem. (Moscow) 2009 , 74 , 1142 .
- Uemura , S. In Comprehensive Organic Synthesis ; Trost , B.M. Fleming , I. ; Pergamon : Oxford , 1991 ; 757 .
- Oae , S. Organic Sulfur Chemistry: Structure and Mechanism ; FL : CRC Press, Boca Raton , 1991 .
- Cremlyn , R.J. An Introduction to Organosulfur Chemistry ; New York : Wiley & Sons , 1996 .
- Maiti , S.N. ; Spevak , P. ; Singh , M.P. ; Micetich , R.G. Synth. Commun . 1988 , 18 , 575 .
- Joshi , A.V. ; Bhusare , S. ; Baidossi , M. ; Qafisheh , N. ; Sasson , Y. Tetrahedron Lett . 2005 , 46 , 3583 .
- Shaabani , A. ; Tavasoli-Rad , F. ; Lee , D.G. Synth. Commun . 2005 , 35 , 571 .
- Ali , M.H. ; McDermott , M. Tetrahedron Lett . 2002 , 43 , 6271 .
- Christoforou , A. ; Nicolaou , G. ; Elemes , Y. Tetrahedron Lett . 2006 , 47 , 9211 .
- Raghavan , S. ; Rajender , A. ; Joseph , S.C. ; Rasheed , M.A. Synth. Commun . 2001 , 31 , 1477 .
- Zhong , P. ; Guo , M.P. Synth. Commun . 2001 , 31 , 1825 .
- Misra , A.K. ; Agnihotri , G. Synth. Commun . 2004 , 34 , 1079 .
- Alam , A. ; Takaguchi , Y. ; Tsuboi , S. Synth. Commun . 2005 , 35 , 1329 .
- Liu , K.T. ; Tong , Y.C. Synthesis 1978 , 669 .
- Shah , S.T.A. ; Khan , K.M. ; Fecker , M. ; Voelter , W. Tetrahedron Lett . 2003 , 44 , 6789 .
- Hirano , M. ; Yakabe , S. ; Fukami , M. ; Morimoto , T. Synth. Commun . 1997 , 27 , 2783 .
- Salehi , P. ; Farrokhi , A. ; Gholizadeh , M. Synth. Commun . 2001 , 31 , 2777 .
- Khazaei , A. ; Zolfigol , M.A. ; Rostami , A. Synthesis 2004 , 2959 .
- Leino , R. ; Lönnqvist , J.E. Tetrahedron Lett . 2004 , 45 , 8489 .
- Demir , A.S. ; Igdir , A.C. ; Mahasneh , A.S. Tetrahedron 1999 , 55 , 12399 .
- Shaabani , A. ; Mirzaei , P. ; Lee , D.G. Catal. Lett . 2004 , 97 , 119 .
- Shaabani , A. ; Bazgir , A. ; Lee , D.G. Synth. Commun . 2004 , 34 , 3595 .
- Silveira , C.C. ; Mendes , S.R. Tetrahedron Lett . 2007 , 48 , 7469 .
- Lenardão , E.J. ; Lara , R.G. ; Silva , M.S. ; Jacob , R.G. ; Perin , G. Tetrahedron Lett . 2007 , 48 , 7668 .
- Thurow , S. ; Pereira , V.A. ; Martinez , D.M. ; Alves , D. ; Perin , G. ; Jacob , R.G. ; Lenardão , E.J. Tetrahedron Lett . 2011 , 52 , 640 .
- Singh , D. ; Galetto , F.Z. ; Soares , L.C. ; Rodrigues , O.E.D. ; Braga , A.L. Eur. J. Org. Chem . 2010 , 2661 .
- Randall , J.B. ; Michael , A.B. ; Evans-White , M.A. ; Lamberti , G.A. Environ. Toxicol. Chem . 2005 , 24 , 87 .
- Ranke , J. ; Stolte , S. ; Störmann , J. ; Arning , J. ; Jastorff , B. Chem. Rev . 2007 , 107 , 2183 .
- Wells , A.S. ; Coombe , V.T. Org. Process Res. Dev . 2006 , 10 , 794 .
- Docherty , K.M. ; Kulpa Jr C.F. Green Chem . 2005 , 7 , 185 .
- Nascimento , J.E.R. ; Barcellos , A.M. ; Sachini , M. ; Perin , G. ; Lenardão , E.J. ; Alves , D. ; Jacob , R.G. ; Missau , F. Tetrahedron Lett . 2011 , 52 , 2571 .
- Alves , D. ; Sachini , M. ; Jacob , R.G. ; Lenardão , E.J. ; Contreira , M.E. ; Savegnago , L. ; Perin , G. Tetrahedron Lett . 2011 , 52 , 133 .
- Gonçalves , L.C. ; Fiss , G.F. ; Perin , G. ; Alves , D. ; Jacob , R.G. ; Lenardão , E.J. Tetrahedron Lett . 2010 , 51 , 6772 .
- Perin , G. ; Mello , L.G. ; Radatz , C.S. ; Savegnago , L. ; Alves , D. ; Jacob , R.G. ; Lenardão , E.J. Tetrahedron Lett . 2010 , 51 , 4354 .
- Silveira , C.C. ; Mendes , S.R. ; Líbero , F.M. ; Lenardão , E.J. ; Perin , G. Tetrahedron Lett . 2009 , 50 , 6060 .
- Lenardão , E.J. ; Feijó , J.O. ; Thurow , S. ; Perin , G. ; Jacob , R.G. ; Silveira , C.C. Tetrahedron Lett . 2009 , 50 , 5215 .
- Victoria , F.N. ; Radatz , C.S. ; Sachini , M. ; Jacob , R.G. ; Perin , G. ; Silva , W.P. ; Lenardão , E.J. Tetrahedron Lett . 2009 , 50 , 6761 .
- Megnerian , G. ; Clapp , L.B. J. Am. Chem. Soc . 1951 , 73 , 486 .
- Barba , F. ; Ranz , F. ; Batanero , B Tetrahedron Lett . 2009 , 50 , 6798 .
- Tajbakhsh , M. ; Habibzadeh , S. J. Chem. Res (S) 2007 , 486 .
- Hajipour , A.J. ; Mallakpour , S.E. J. Chem. Res. (S) 2000 , 32 .
- Iranpoor , N. ; Zeynizadeh , B. Synthesis 1999 , 49 .
- Khan , K.M. ; Taha , M. ; Rahim , F. ; Ali , M. ; Jamil , W. ; Perveen , S. ; Choudhary , M.I. Lett. Org. Chem . 2010 , 7 , 415 .
- Field , L. ; Lawson , J.E. J. Am. Chem. Soc . 1958 , 80 , 838 .
- Banfield , S.C. ; Omori , A.T. ; Leisch , H. ; Hudlicky , T. J. Org. Chem . 2007 , 72 , 4989 .
- Hergett , S.C. ; Peach , M.E. J. Fluorine Chem . 1988 , 38 , 367 .
- Collings , A.J. ; Morgan , K.J. Tetrahedron 1964 , 20 , 2167 .
- Ruano , J.L.G. ; Parra , A. ; Alemán , J. Green Chem . 2008 , 10 , 706 .
- Wang , X. ; Chen , X. ; Wu , X. ; Tao , J. ; Cheng , L. Synth. Commun . 2009 , 39 , 3453 .
- Xiao , H. ; Chen , J. ; Liu , M. ; Wu , H. ; Ding , J. Phosphorus Sulfur Silicon 2009 , 184 , 2553 . http://www.informaworld.com/smpp/title~db=all~content =t713618290~tab=issueslist~branches=184-v184
- Kirihara , M. ; Asai , Y. ; Ogawa , S. ; Noguchi , T. ; Hatano , A. ; Hirai , Y. Synthesis 2007 , 3286 .
- Meshram , H.M. Org. Prep. Proced. Int . 1993 , 25 , 232 .
- Badri , R. ; Mostoufi , A. Phosphorus Sulfur Silicon 2006 , 181 , 1513 .
- Rai , S.K. ; Sharma , M. ; Tiwari , M. Bioorg. Med. Chem . 2008 , 16 , 7302 .