Abstract
We have reported an efficient and eco-friendly protocol for the protection of various structurally and electronically divergent aryl and aliphatic amines using Cbz-Cl in the presence of polyethylene glycol (PEG)-400 at room temperature. The reaction afforded excellent yields with low as well as high molecular weight PEGs.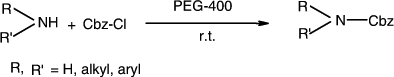
Introduction
In recent years, the development of eco-friendly organic syntheses is gaining considerable interest both in industrial and academic research Citation1. Hazardous, toxic, and volatile organic solvents are being continuously replaced by the use of solvent-free techniques Citation2, water Citation3, phase-transfer catalysts, or ionic liquids Citation4. The use of polyethylene glycol (PEG) as a reaction medium is highly beneficial as the system remains neutral, which helps in maintaining a number of acid- and base-sensitive functional groups remains unchanged Citation5.
Protection and deprotection of functional groups are important and frequently needed exercises in organic chemistry. Protection of amines is particularly very important due to their high nucleophilicity and basicity. Among the widely used protecting groups for amines, the benzyloxycarbonyl (Cbz) group is extensively used because it can be easily removed by catalytic hydrogenation Citation6–9. Furthermore, the Cbz group is stable toward basic and most aqueous acidic conditions Citation10. Generally N-Cbz protection of amines is carried out by the treatment of amines with benzyloxycarbonyl chloride (Cbz-Cl) in the presence of 4-(dimethylamino)pyridine or organic/inorganic bases Citation10. Recently, a few methods using β-cyclodextrin Citation11, molecular iodine Citation12 , tetrabutylammonium bromide Citation13, and polymer-bound 1-hydroxybenzotriazole Citation14 has been reported. However, these methods have various drawbacks such as requirement of anhydrous solvents and formation of side products. These drawbacks necessitate the development of efficient new synthetic methodologies.
In general, PEG and their monomethyl ethers are inexpensive, thermally stable, non-toxic Citation15, and are widely used in food products Citation16 as well as in cosmetics Citation17 . PEG is water miscible which facilitates its removal from reaction products. Recently, great attention has been focused on the use of PEG as a benign, alternative reaction medium in the synthesis of various organic compounds Citation5 Citation18–28.
Results and discussion
In continuation of our investigations Citation29–32 in the development of new synthetic methodologies for carbon-heteroatom bond formation, herein we report an efficient and eco-friendly protocol for the protection of amines using Cbz-Cl in the presence of PEG-400 at room temperature ().
To optimize the reaction conditions, we investigated the reaction with aniline (1 mmol), benzyloxycarbonyl chloride (Cbz-Cl) (1 mmol), and different quantities of PEG-400. Furthermore, we studied this reaction using various solvents including tap water in the presence of PEG-400. The optimized results are summarized in . We found that the best result was obtained with 0.5 mL of PEG-400 for 1 mmol of aniline in the absence of any solvent at room temperature within 5 min (, entry 4). Using larger amounts of PEG-400 did not improve the yield of the product. In addition, low yield of the product was obtained in the absence of PEG-400 (, entry 1). Thus in this reaction 0.5 mL of PEG-400 acted as a good promoter. We investigated our protocol with PEGs of various molecular weights 200, 400, 600, 4000, and 6000 (0.05 mol% each) for our model reaction with aniline (1 mmol) and benzyloxycarbonyl chloride (Cbz-Cl) (1 mmol). The reaction afforded excellent yields with low as well as high molecular weight PEGs. With the above results in hand, a variety of amines such as aliphatic, aromatic, and heterocyclic were employed for N-Cbz protection. It was found that the yields were excellent in all the cases (). This protocol is highly chemoselective as the amine group is only protected even in the presence of OH/SH groups and mono N-Cbz protected products were obtained in excellent yields.
Table 1. N-Cbz protection of aniline with variety of polyethylene glycols (PEGs) in various conditions at room temperature (entry 3 gives the optimum conditions).
Experimental
General experimental procedure for N-Cbz protection of amines
To a magnetically stirred mixture of Cbz-Cl (1 mmol) and PEG-400 (0.5 mL), amine (1 mmol) was added at room temperature. After stirring the reaction mixture for a specified time (), diethyl ether (20 ml) was added to the reaction mixture. The organic phase was separated and washed aqueous saturated NaHCO3 (5 ml), dried over anhydrous sodium sulfate and evaporated. The crude product was purified by column chromatography on silica gel using hexane-ethyl acetate (7:3) as eluent. All the known compounds were characterized by comparing their physical and spectral data with those reported Citation10–13.
Table 2. PEG-400 mediated N-Cbz protection of amines at room temperature.
The data for the selected N-Cbz products (, entries 10, 13, and 18) are given below.
(4-Hydroxy-phenyl)-carbamic acid benzylester (Entry10, )
Mp: 138–140°C; FT-IR (KBr): 3332, 3082, 2952, 2870, 1732, 1605, 1583, 1510, 1380, 1326,1237, 1027, 635 cm–1; 1H NMR (400 MHz, CDCl3+ DMSO-d 6): 8.56 (brs, 1H), 7.22–7.35 (m, 5H), 7.21 (d, J=8.1 Hz, 2H), 6.71 (d, J=8.1 Hz, 2H), 5.14 (s, 2H), 3.62 (s, 1H); 13C NMR (100 MHz, CDCl3+ DMSO-d 6): 65.2, 114.6, 119.9, 127.2, 127.5, 129.7, 135.8, 152.3, 153.2; ESI-MS=242 (M-H)+; Anal. Calcd for C14H13NO3: C, 69.12; H, 5.39; N, 5.76; Found: C, 69.07; H, 5.42; N, 5.70.
(4-Acetyl-phenyl)-carbamic acid benzy ester (Entry13, )
Mp: 125–127°C; FT-IR (KBr): 3323, 3200, 2930, 2870, 1721, 1662, 1601, 1585, 1521, 1290, 1045, 745 cm–1; 1H NMR (400 MHz, CDCl3): 7.91 (d, J=8.8 Hz, 2H), 7.48 (d, J=8.8 Hz, 2H), 7.33–7.40 (m, 5H), 7.06 (s, 1H), 5.20 (s, 2H); 13C NMR (100 MHz, CDCl3): 26.3, 67.1, 117.7, 128.8, 129.7, 132.0, 135.6, 142.4, 152.9, 196.9; ESI-MS: m/z=268 (M-H)+; Anal. Calcd for C16H15NO3: C, 71.36; H, 5.61; N, 5.20; Found: C, 71.31; H, 5.66; N, 5.15.
Benzyloxycarbonylamino-acetic acid methylester (Entry18, )
FT-IR (KBr): 3092, 2930, 2893, 1746, 1711, 1605, 1585, 1472, 1290, 1045, 625 cm–1; 1H NMR (400 MHz, CDCl3): 7.29–7.21 (m, 5H), 5.83 (brs, 1H), 5.03 (s, 2H), 4.08 (q, J=7.2 Hz, 2H), 3.82 (d, J=5.6 Hz), 1.15 (t, J=7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3): 13.6, 42.3, 60.8, 66.4, 127.5, 128.0, 136.0, 156.1, 169.7; ESI-MS: m/z=236 (M-H)+; Anal. Calcd for C12H15NO4: C, 60.75; H, 6.37; N, 5.90; Found: C, 60.69; H, 6.42; N, 5.87.
Conclusion
In conclusion, we have developed a simple and efficient protocol for N-Cbz protection of amines in high yields in the presence of PEG-400 at room temperature. In this reaction, PEG-400 acted as an efficient “green” promoter. Therefore, this is a versatile and environmentally benign eco-friendly procedure that proves beneficial to both academic and industrial fields.
Acknowledgements
We gratefully acknowledge the financial support from the Department of Science and Technology (DST), New Delhi.
References
- Clark , J. and Macquarrie , D.M.A. 2002 . Handbook of Green Chemistry , 1 – 531 . Blackwell : Oxford .
- Tanaka , K. Solvent-Free Organic Synthesis ; Wiley-VCH Weinheim 2003 ; pp 1 – 402 .
- Li , C.J. 2005 . Organic Reactions in Aqueous Media with a Focus on Carbon–Carbon Bond Formations: A Decade Update . Chem. Rev , 105 : 3095 – 3165 .
- Koichi , M. Green Reaction Media for Organic Synthesis ; Wiley–VCH Weiheim 2005 ; pp 9 – 58 .
- Jain , L.S. , Singhal , S. and Sain , B. 2007 . PEG Assisted Solvent and Catalyst free Synthesis of 3,4-dihydropyrimidinones under Mild Reaction Conditions . Green Chem , 9 : 740 – 741 .
- Fieser , L.F. and Fieser , M. 1967 . In Reagents for Organic Synthesis, Vol. 1 , 109 New York : John Wiley & Sons .
- Berkowitz , D.B. and Pedersen , M.L. 1994 . Simultaneous Amino and Carboxyl Group Protection for.alpha.-Branched Amino Acids . J. Org. Chem , 59 : 5476 – 5478 .
- Maligres , P.E. , Houpis , I. , Rossen , K. , Molina , A. , Sager , J. , Upadhyay , V. , Wells , K.M. , Reamer , R.A. , Lynch , J.E. , Askin , D. , Volante , R.P. and Reider , P.J. 1997 . Synthesis of the Orally active Spiroindoline-based Growth Hormone Secretagogue, MK-677 . Tetrahedron , 32 : 10983 – 10992 .
- Hernandez , J.N. and Martin , V.S. 2004 . First Practical Protection of (-Amino Acids as N,N-Benzyloxycarbamoyl Derivatives . J. Org. Chem , 69 : 3590 – 3592 .
- Greene , T.W. and Wuts , P.G.M. 1998 . Protective Groups in Organic Synthesis , 531 – 539 . Wiley : New York .
- Kumar , P.V. , Reddy , M.S. , Narender , M. , Surendra , K. , Nageswar , Y.V. D. and Rao , K.R. 2006 . Aqueous Phase Mono-Protection of Amines and Amino Acids as N-benzyloxycarbonyl Derivatives in the Presence of β-Cyclodextrin . Tetrahedron Lett , 47 : 6393 – 6396 .
- Varala , R. , Enugala , R. and Adapa , S.R. 2007 . Molecular Iodine-Catalyzed Efficient N-Cbz Protection of Amines . J. Iran. Chem. Soc , 4 : 370 – 374 .
- Suresh Babu , K. , Subba Rao , V.R. , Ranga Rao , R. , Sivaram Babu , S. and Madhusudana Rao , J. 2009 . A Mild and Efficient Chemoselective N-Benzyloxycarbonylation of Amines using TBAB as a Catalyst under Solvent-Free Conditions . Can. J. Chem , 87 : 393 – 396 .
- Dendrinos , K.G. and Kalivretenos , A.G. 1998 . Convenient Protection of Amines as Carbamates using Polymer-bound HOBT as Catalyst . J. Chem. Soc. Perkin Trans , 1 : 1463 – 1464 .
- Chem , J. , Spear , S.K. , Huddleston , J.G. and Rogers , R.D. 2005 . Polyethylene Glycol and Solutions of Polyethylene Glycol as Green Reaction Media . Green Chem. , 7 : 64 – 82 .
- Sheftel , V.O. Indirect Food Additives and Polymers: Migration and Toxicology . Lewis 2000 , 1114 – 1116 .
- Claudia , F.-P. 2005 . Safety Assessment on Polyethylene Glycol (PEGs) and their Derivatives as used in Cosmetic Products . Toxicology , 214 : 1 – 38 .
- Vasudevan , V.N. and Varma , R.S. 2001 . Microwave-accelerated Suzuki cross-Coupling Reaction in Polyethylene Glycol (PEG) . Green Chem , 3 : 146 – 148 .
- Chandrasekhar , S. , Narsihmulu , C. , Sultana , S.S. and Reddy , N.R. 2002 . Poly(ethylene glycol) (PEG) as a Reusable Solvent Medium for Organic Synthesis. Application in the Heck Reaction . Organic Lett , 4 : 4399 – 4401 .
- Haimov , A. ; Newmann , R. Polyethylene Glycol as a non-ionic Liquid Solvent for Polyoxometalate Catalyzed Aerobic Oxidation . Chem. Commun . 2002 , 876 – 877 .
- Mukhopadhyay , C. and Tapaswi , P.K. 2008 . PEG-mediated Catalyst-Free Expeditious Synthesis of 2-substituted Benzimidazoles and Bis-Benzimidazoles under Solvent-less Conditions . Tetrahedron Lett , 49 : 6237 – 6240 .
- He , F. , Li , S. , Vert , M. and Zhuo , R. 2003 . Enzyme-Catalyzed Polymerization and Degradation of Copolymers Prepared from ϵ- Caprolactone and Poly(ethylene glycol) . Polymer , 44 : 5145 – 5151 .
- Wei , W. , Wang , Y.H. , Jiang , J.Y. and Jin , Z.L. 2007 . A Novel Phosphate Ligand Used for the Rh-catalyzed Hydroformylation of Cyclohexene in a Thermoregulated PEG Biphase System . Chinees Chem. Lett , 8 : 933 – 935 .
- Kidwai , M. , Bhatnagar , D. and Neeraj , K.M. 2010 . Polyethylene Glycol (PEG) Mediated Green Synthesis of 2,5-Disubstituted 1,3,4-Oxadiazoles Catalyzed by Ceric Ammonium Nitrate (CAN) . Green Chem. Lett. Rev , 3 : 55 – 59 .
- Kidwai , M. , Mishra , N.K. , Bhatnagar , D. and Jahan , A. 2011 . A Green Methodology for One-Pot Synthesis of Polysubstituted-Tetrahydropyrimidines using PEG . Green Chem. Lett. Rev , 4 : 109 – 115 .
- Kidwai , M.. and Bhatnagar , D. 2010 . Ceric Ammonium Nitrate (CAN) Catalyzed Synthesis of N-substituted Decahydroacridine- 1,8-diones in PEG . Tetrahedron Lett , 51 : 2700 – 2703 .
- Kidwai , M. , Jahan , A. and Bhatnagar , D. 2010 . Polyethylene Glycol as an Efficient and Reusable Solvent Medium for the Synthesis of Thiohydantoins using K2CO3 as Catalyst . J. Sulf. Chem , 31 : 161 – 167 .
- Kidwai , M. , Jahan , A. and Bhatnagar , D. 2010 . Polyethylene Glycol: A Recyclable Solvent System for the Synthesis of Benzimidazole Derivatives using CAN as Catalyst . J. Chem. Sci , 122 : 607 – 612 .
- Maheswara , M. , Siddaiah , V. , Damu , G.L.V. and Venkata Rao , C. 2006 . An Efficient One-pot Synthesis of Polyhydroquinoline Derivatives via Hantzsch Condensation using a Heterogeneous Catalyst under Solvent-Free Conditions . ARKIVOC , ii : 201 – 206 .
- Maheswara , M. ; Siddaiah , V. ; Gopalaiah , K. ; Madhava Rao , V. ; Venkata Rao , C. A Simple and Effective Glycine-Catalyzed Procedure for the Preparation of Oximes . J. Chem. Res . June 2006 , 362 – 363 .
- Maheswara , M. , Siddaiah , V. , Koteswara Rao , Y. , Yew-Min Tzeng , Y.-T. and Sridhar , C. 2006 . A Simple and Efficient One-Pot Synthesis of 1,4-Dihydropyridines Using Heterogeneous Catalyst under Solvent-Free Conditions . J. Mol. Cat. A: Chemical , 260 : 179 – 180 .
- Siddaiah , V. , Basha , G.M. , Padma Rao , G. , Viplava Prasad , U. and Suryachendra Rao , R. 2010 . PEG-mediated Facile Protocol for N-Boc Protection of Amines . Chem. Lett , 39 : 1127 – 1129 .
