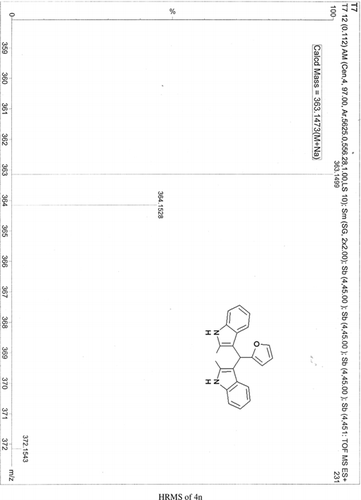
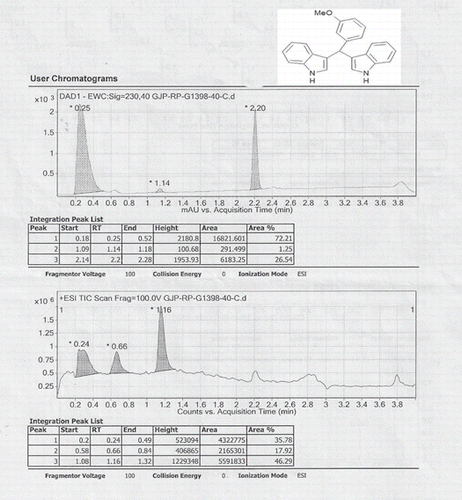
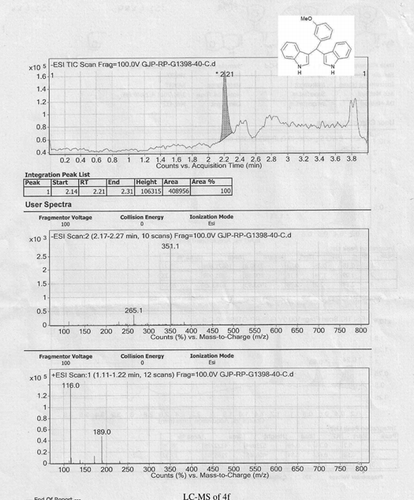
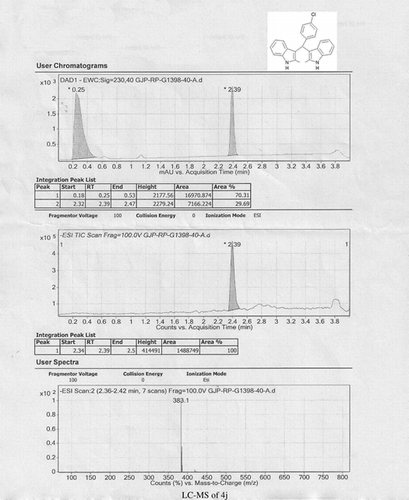
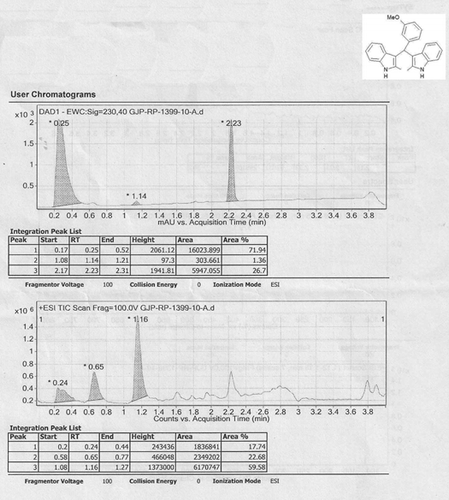
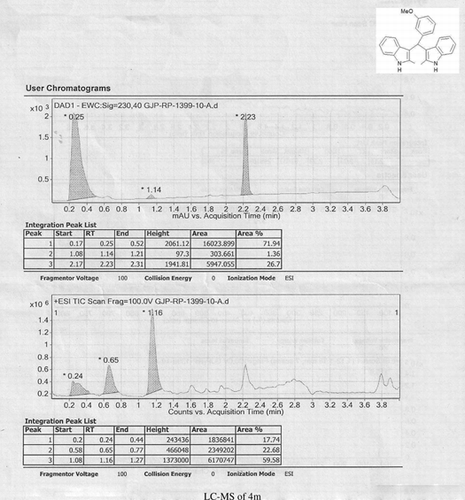
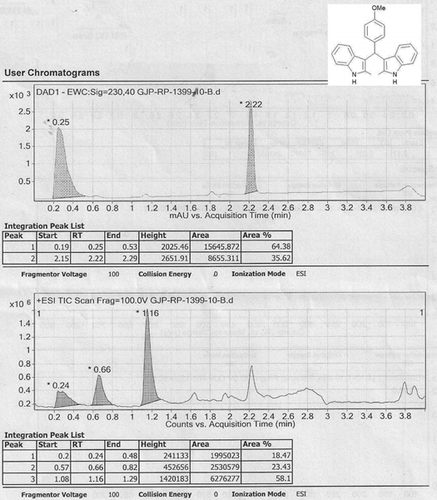
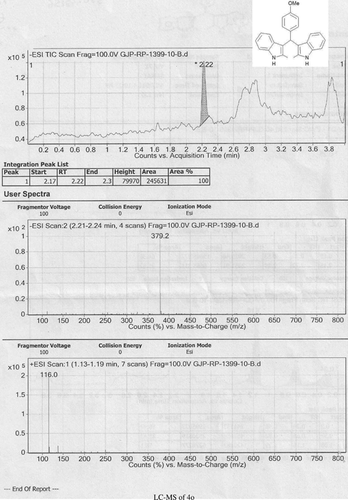
Abstract
Condensation of indole with aromatic aldehydes has been carried out without using any catalyst and solvent to give bis(indolyl)methanes.
Introduction
The focus of green chemistry lies on reducing environmental pollution by designing benign reaction processes, avoiding waste generation, and saving energy by reducing number of chemical steps. Multicomponent reactions Citation1–3 and domino reactions Citation4–15 are the fascinating offshoots of the search for reducing chemical steps. Solventless or when solvent is a must, water as a preferred medium and use of catalyst or catalyst-free reaction conditions are some of the solutions to avoid waste. Bis(indolyl)methanes (BIMs) have been attractive targets to develop green methodologies because of their wide range of biological, industrial, and synthetic applications Citation16. (See Supporting information in 16).
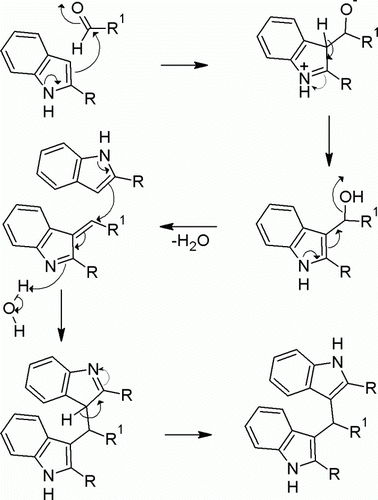
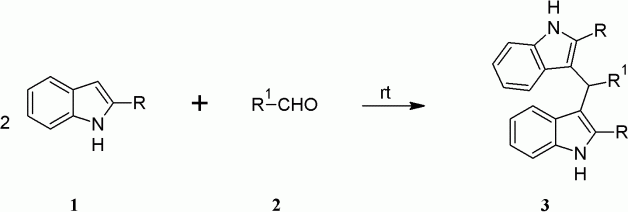
The reaction of indole with aldehydes or ketones produces azafulven, which then undergo further addition with another indole molecule to give BIM and water (). Both protic and Lewis acids are known to promote this reaction. However, many Lewis acids are prone to undergo change in the presence of nitrogen containing reactants and this necessitates the use of excess and sometimes stoichiometric amount of Lewis acid catalyst. The various strategies adopted for the synthesis of BIMs and tris(indolyl)methanes (TIMs) have recently been comprehensively reviewed Citation16. There is continuing interest for the development of a new, practical, economical, and environmental friendly protocol for the synthesis of BIMs. Recent catalysts used for this propose are FeCl3.6H2O Citation17, CuBr Citation18, CeCl3.7H2O Citation19, silica-bonded S-sulfonic acid Citation20, N,N,N′,N′–tetrabromobenzene-1,3-disulfonamide (TBBDA) and poly(N-bromobenzene-1,3-disulfonamide) (PBBS) Citation21, cellulose sulfuric acid Citation22 and ionic liquid 3-methyl-1-sulfonic acid imidazolium chloride Citation23, polystyrene-supported aluminum chloride (PS–AlCl3) Citation24, heteropoly acids Citation25 Citation26, etc.
A perfectly matching green chemistry protocol would be to carry out the synthesis successfully without the use of any catalyst and solvent. To our knowledge, there are no such reports for the synthesis of BIMs. The only report of catalyst free formation of BIMs is in glycerol Citation27 at 90 °C. We disclose herein our findings of solventless and catalyst free synthesis of BIMs.
Results and discussion
The propensity with which indole undergoes electrophilic substitution made us to envisage that, for many of the BIMs reported particularly with aryl aldehydes having electron withdrawing groups, there may not be any need of assistance from promoters like protic acids or catalyst. To check this hypothesis, in the first experiment, we mixed o-chlorobenzaldehyde and indole (1:2 mole ratio) and kept at ambient temperature in a test tube (Method 1). To our expectation, the reaction was found to complete within 45 min Thin Layer Chromatography (TLC). In another experiment a mixture of o-chlorobenzaldehyde and indole (1:2 mole ratio) was ground using mortar and pestle (Method 2) (mechanochemical synthesis) Citation28 Citation29. The reaction was found to complete within 15 min TLC, in shorter time than that in the above mixing experiment. Similar procedure was followed for other aryl aldehydes. Aryl aldehyde with an electron donating group at meta position (Entry f) also underwent reaction. Heterocyclic aldehydes (Entry g, h) also reacted successfully. Expectedly, anisaldehyde with electron donating group at para position and heptaldehyde failed to react completely. Ketones did not react at all, while 2-methylindole reacted rapidly to give corresponding BIMs (Entry i–p). Even anisaldehyde which failed to give significant product with parent indole gave corresponding BIM product with 2-methylindole (Entry p). To check whether the byproduct water () was promoting the reaction, deliberately 2–4 drops of water were added to the mixture. However, no enhancement was observed in the rate of reaction, product formation or yield. Another possibility of catalysis by the trace amount of carboxylic acid formed by aerial oxidation of aryl aldehydes cannot be ruled out.
Experimental
Method 1
Aryl aldehyde (1 eqiv.) and indole (2 eqiv.) were mixed thoroughly in a test tube and kept at ambient temperature in a test tube for a certain period as stipulated in . The crude product obtained was recrystallized with chloroform-ethyl acetate mixture.
Table 1. Synthesis of bis(indolyl)methanes
Method 2
Aryl aldehyde (1 eqiv.) and indole (2 eqiv.) were mixed and ground using mortar and pestle. For compounds having reaction time of more than 15 min, the initial grinding was done for 15 min and the reaction mixture was kept at ambient temperature. The mixture was ground periodically for a short time after every 30 min till the reaction completed as stipulated in . The crude product obtained was recrystallized with chloroform-ethyl acetate mixture.
Conclusion
In conclusion, we have accomplished a green protocol for solvent free and catalyst free synthesis of BIMs from indole/2-methylindole and aryl adehydes.
Acknowledgements
The authors thank DST, New Delhi for financial assistance. One of us (KLD) thanks CSIR, New Delhi for award of JRF.
References
- Toure , B.B. Hall , D.G. Chem. Rev . 2009 , 109 , 4439 – 4486 .
- Sapi , J. ; Laronze , J.Y. ARKIVOC 2004 , vii , 208 – 222 .
- Zhu , J. Bienaymé , H. Multicomponent Reactions Weinheim Wiley-VCH , 2005 .
- Tietze , L.F. Chem. Rev . 1996 , 96 , 115 – 136 .
- Posner , G.H. Chem. Rev . 1986 , 86 , 831 – 844 .
- Jasperse , C.P. Curran , D.P. Fevig , T.L. Chem. Rev . 1991 , 91 , 1237 – 1286 .
- Nicolaou , K.C. Edmonds , D.J. Bulger , P.G. Angew. Chem. Int. Ed . 2006 , 45 , 7134 – 7186 .
- Kirsch , S.F. Synthesis 2008 , 20 , 3183 – 3204 .
- Tietze , L.F. Beifuss , U. Angew. Chem. Int. Ed . 1993 , 32 , 131 – 163 .
- Tietze , L.F. Lieb , M.E. Curr. Opin. Chem. Biol . 1998 , 2 , 363 – 371 .
- Tietze , L.F. Modi , A. Med. Res. Rev . 2000 , 20 , 304 – 322 .
- Tietze , L.F. Brasche , G. Gericke , G. Domino Reactions in Organic Synthesis Wiley-VCH : Weinheim , 2006 .
- Parsons , P.J. Penkett , C.S. Shell , A.J. Chem. Rev . 1996 , 96 , 195 – 206 .
- Ho , T.L. Tandem Reactions in Organic Synthesis ; New York : Wiely Interscience , 1997 .
- Taylor , R.J.K. Reid , M. Foot , J. Raw , S.A. Acc. Chem. Res . 2005 , 38 , 851 – 869 .
- Shiri , M. Zolfigol , M.A. Kruger , H.G. Tanbakouchian , Z. Chem. Rev . 2010 , 110 , 2250 – 2293 .
- Thirupathi , P. Kim , S.S. J. Org. Chem . 2010 , 75 15 , 5240 – 5249 .
- Yang , J. Wang , Z. Pan , F. Li , Y. Bao , W. Org. Biomol. Chem . 2010 , 8 13 , 2975 – 2978 .
- Das , B. Kumar , R.A. Aruna , D. Kashanna , J. Indian J. Heterocycl. Chem . 2010 , 19 3 , 295 – 296 .
- Niknam , K. Saberi , D. Baghernejad , M. Phosphorus, Sulfur, and Silicon 2010 , 185 4 , 875 – 882 .
- Ghorbani-Vaghei , R. Malaekehpoor , S.M. Org. Prep. Proced. Int . 2010 , 42 2 , 175 – 182 .
- Alinezhad , H. Haghighi , A.H. Salehian , F. Chin. Chem. Lett . 2010 , 21 2 , 183 – 186 .
- Zolfigol , M.A. Khazaei , A. Moosavi-Zare , A.R. Zare , A. Org. Prep. Proced. Int . 2010 , 42 1 , 95 – 102 .
- Tamami , B. Shirazi , A.N. Borujeni , K.P. J. Serb. Chem. Soc . 2010 , 75 4 , 423 – 431 .
- Li , J.-T. Zhang , X.-H. Song , Y.-L. Int. J. ChemTech. Res . 2010 , 2 1 , 341 – 345 .
- Heravi , M.M. Sadjadi , S. J. Iran. Chem. Soc . 2009 , 6 1 , 1 – 54 .
- He , F. Li , P. Gu , Y. Li , G. Green Chem . 2009 , 11 , 1767 – 1773 .
- Lyakhov , N.Z. Grigorieva , T.F. Barinova , A.P. Vorsina , I.A. Russ. Chem. Rev . 2010 , 79 , 189 – 203 .
- Dushkin , A.V. Chem. Sust. Dev . 2004 , 12 , 251 – 273 .
Supporting information
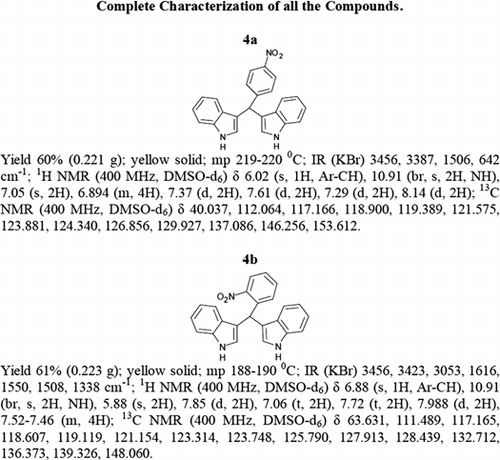
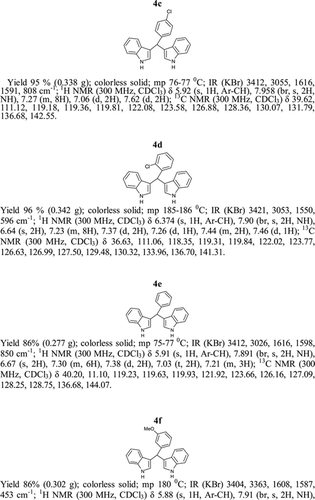
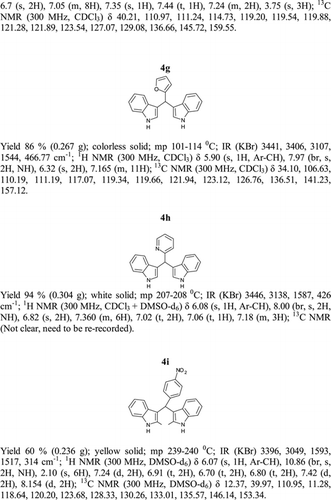
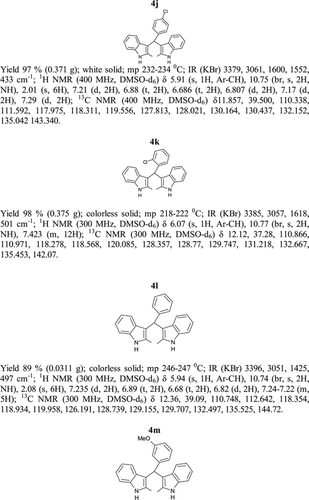
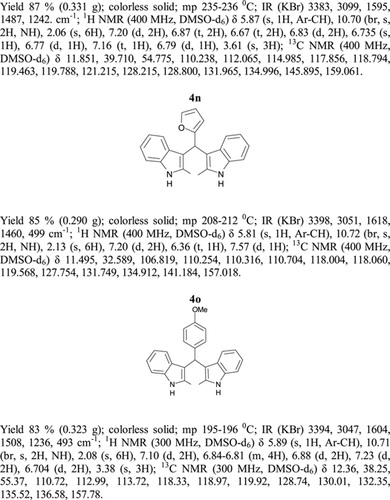
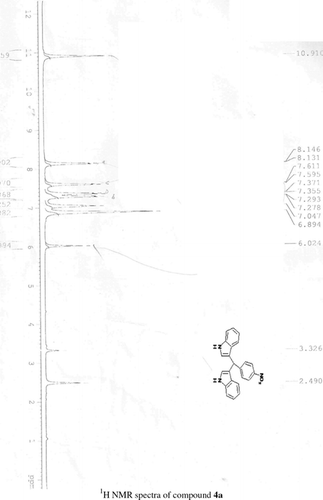
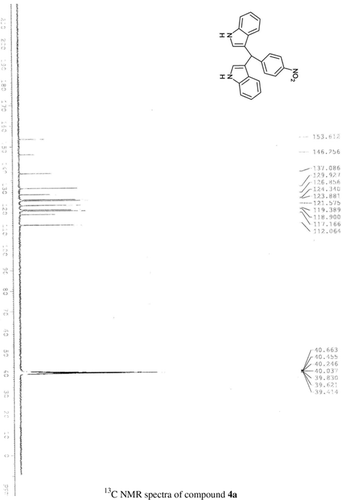
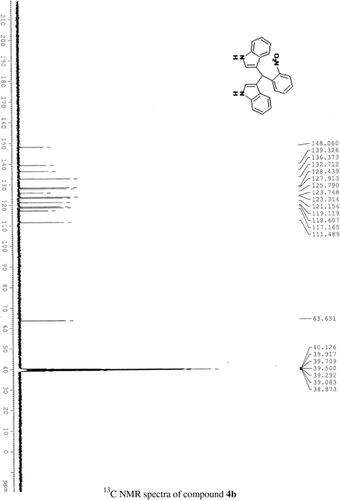
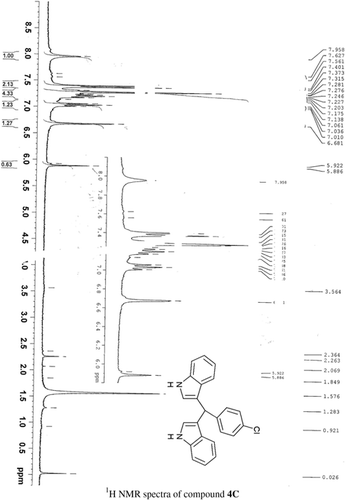
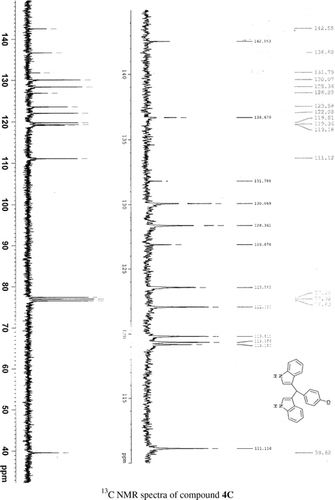
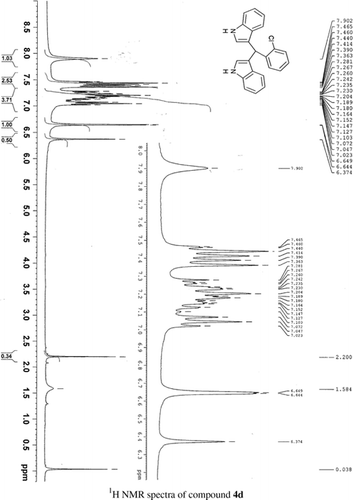
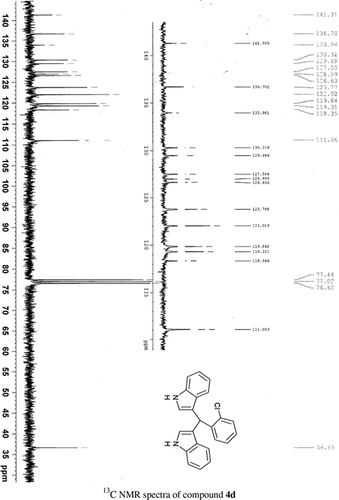
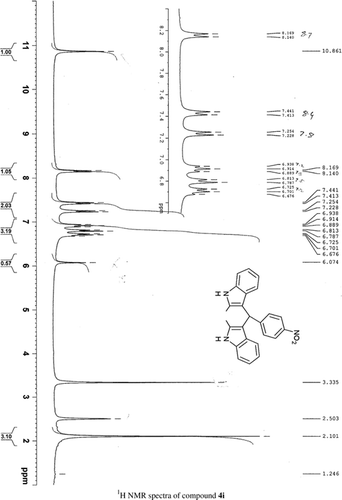
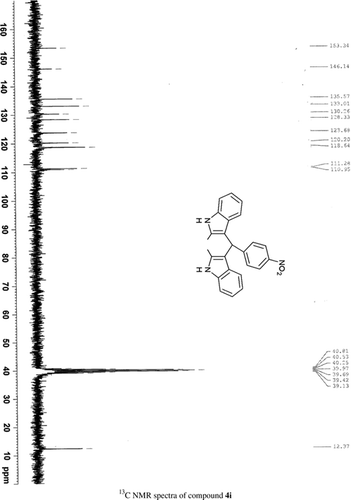
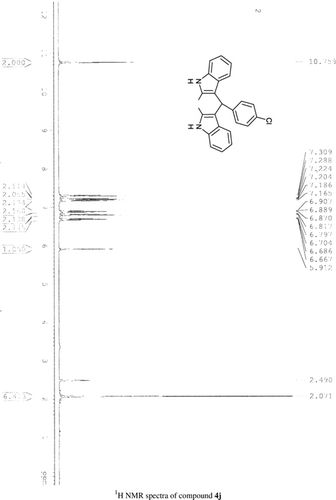
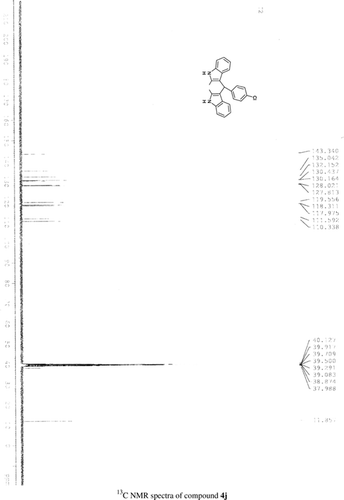
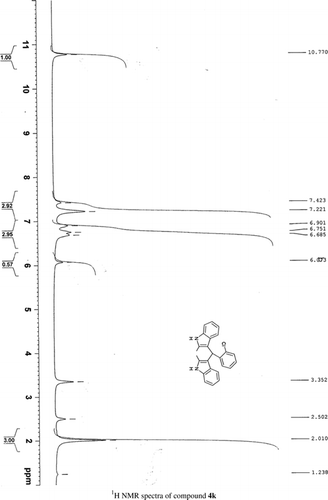
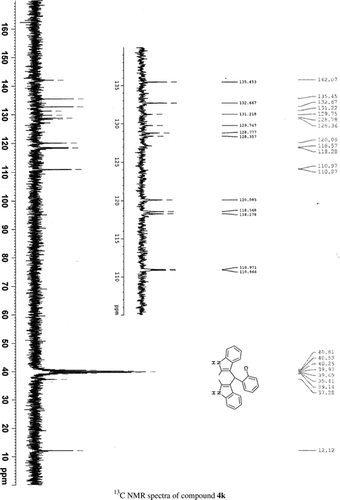
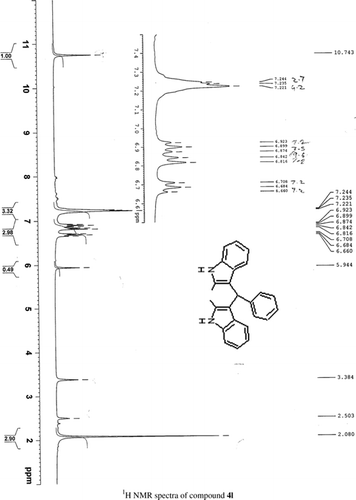
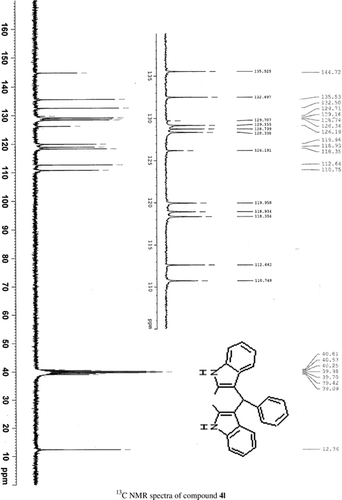
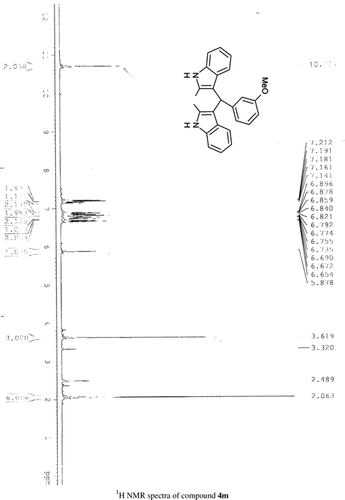
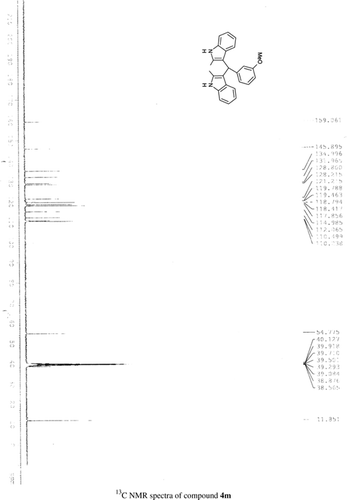
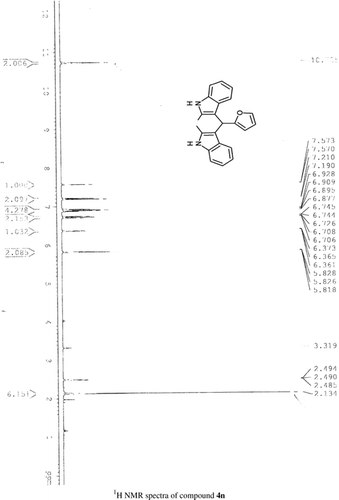
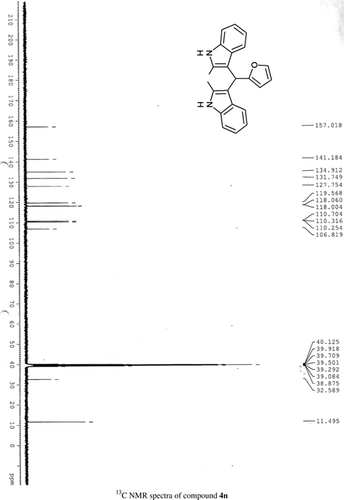
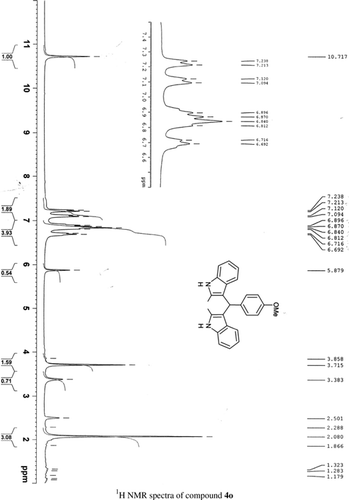
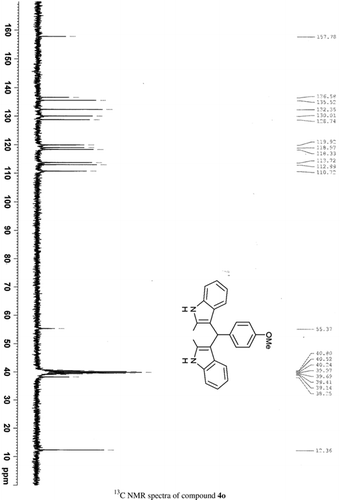
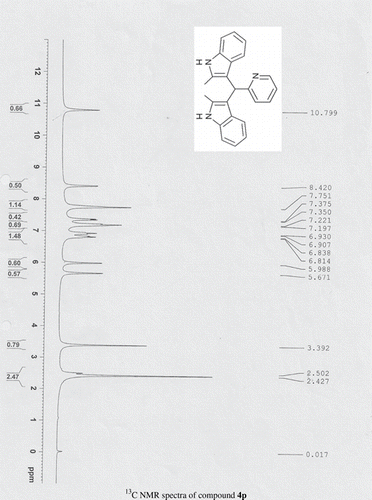
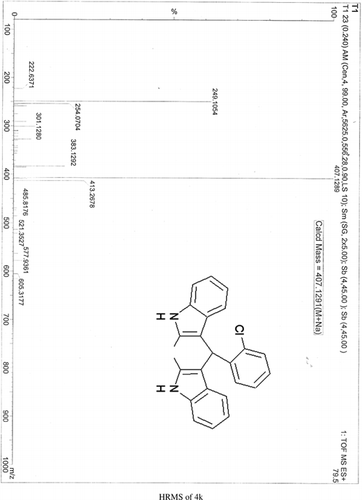
General methods. Commercial reagents were used without further purification. 1H NMR (300 and 400 MHz) and 13C NMR (300 and 400 MHz) were recorded using CDCl3 and DMSO-d6 as solvent and TMS as an internal standard. IR spectra's were recorded in KBr. Melting points are uncorrected.
General procedure; Method-1; Indole (1) (2 mmol)/2-methyl indole (2) (2 mmol), aryl aldehydes (1a-1p) (1 mmol) were mixed thoroughly in a test tube and kept at ambient temprature. The reaction was monitored using TLC. The crude product was recrystallised using chloroform and ethyla cetate mixture.
Method-2; Indole (1) (2 mmol)/2-methyl indole (2) (2 mmol), aryl aldehydes (1a-1p) (1 mmol) was ground using mortar and pastle. The reaction was monitored by TLC. For compounds requiring more than 15 min, the grinding was done intermittently after every 30 min. The crude product was then recrystallised using chloroform and EtOAc.