Abstract
The synthesis of diamino triphenyl methanes from aniline and aromatic aldehydes was conducted in near critical water and supercritical water. The reaction parameters, such as temperature, density, and reaction time, have been studied. Significant acceleration of the condensation reaction of aniline and aromatic aldehydes can be achieved by using high temperature water, especially near the critical point, in the absence of any acid catalysts. It has been demonstrated that high temperature water act effectively in the place of conventional acid catalysts.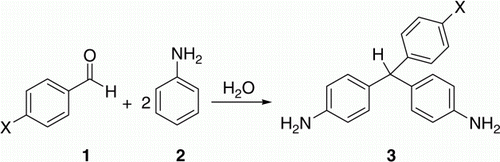
Introduction
Triphenyl methane is a very important class of organic compound with a wide range of applications. Some derivatives of triphenyl methane exhibit biological properties such as antifungal, Host–guest chemistry, material science, and high performance polymers are the areas in which these compounds are used as precursors Citation1.
Considering the influence of chemicals on the environment, investigating environment-friendly approaches to chemical processes seems to be of high necessity. Substituting organic solvents by environmental fluids such as water can decrease the toxicity and the risk management of chemical processes. Supercritical fluids have many environmental and technological advantages, and much attention has been drawn to organic syntheses in supercritical fluids Citation2.
The advantage of water is that it is abundant, cheap, non-toxic, and neither combustible nor explosive. Under supercritical conditions, supercritical water (SCW) behaves very differently from water at ambient temperatures. It is “organiclike” because of the decrease in its dielectric constant, and miscible with gases and many organic substances, thereby overcoming mass transfer limitations which could occur across phase boundaries. SCW can also act as a catalyst in acid based catalyzed reactions or as a reagent in hydrolysis reactions. There are a number of cases in which, water plays three different roles at the same time: as a solvent, as a reactant, and as a catalyst Citation3.
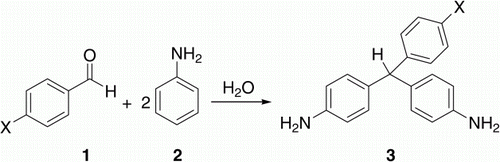
In this paper we attempted to conduct noncatalytic condensation of aniline with various aromatic aldehydes using near critical water (NCW) and supercritical water (SCW). It will be shown that high reaction rate and high yield are possible using NCW and SCW as an alternative, environmentally benign solvent ().
Experimental
Experiments were conducted with stainless steel high pressure resistant reactor whose internal volume was 5 cm3. One side of the tube served as a thermocouple well for measuring the reactor temperature, and the other side was sealed. In the experiments, the reactor was charged with a 1:2 molar ratio of aldehydes and aniline and certain amount of water. The amount of water ranged from 0.3 to 2 g, corresponding to a solution density range of 0.06–0.40 g/cm3. To maintain the safety margin, the 5 cm3 stainless steel autoclave was loaded by only 3 cm3 of the solution. The air in the reactor was replaced with nitrogen via successive purging, and the reactor was sealed. The reactor was heated to 100–400 °C. Reaction times did not include the time taken to heat the contents. After the desired reaction time had elapsed, the reactor was rapidly quenched in water bath and the products were extracted with dichloromethane. All compounds were known and their physical and spectroscopic data were compared with those of authentic samples and found to be identical Citation1, Citation4.
2.1. Physical and spectral data for selected compounds
4,4′-Diaminotriphenylmethane (1b): mp: 130–131 °C (lit.[4] mp: 126); FT-IR (KBr) 3456, 3356(NH2), 3021(=CH), 2821(CH),1621(C=C) cm−1; 1H NMR (300 MHz, CDCl3, δ, ppm) 3.6 (brs, 4H), 5.34 (s, 1H), 6.46,6.81 (AB system, 8H), 7.06–7.24 (m, 4H); MS: m/z 274 [M+], 197, 180, 165, 93, 58, 43.
4,4′-Diamino-4′′-nitrotriphenylmethane (1d): mp: 84–85 °C (lit.[4] mp: 83–84); FT-IR (KBr) 3440, 3356 (NH2), 3100(=CH), 2792(CH), 1622(C=C), 1344–1450(NO2) cm−1; 1H NMR (300 MHz, CDCl3, δ, ppm) 3.6 (brs, 4H), 5.39 (s, 1H), 6.43, 6.81(AB system, 8H), 7.32, 8.08 (AB system); MS: m/z 319 [M+], 292, 197, 180, 93, 65.
4,4′-Diamino-4′′-methoxytriphenylmethane (1e): mp: 125–126 °C (lit.[4] mp: 126–127); FT-IR (KBr) 3412, 3336 (NH2), 3021(=CH), 2958(CH), 1608(C=C), 1026(C–O) cm−1; 1H NMR (300 MHz, CDCl3, δ, ppm) 3.5 (brs, 4H), 3.74(s, 3H), 5.34 (s, 1H), 6.43, 6.83 (AB system, 8H), 6.81, 7.03 (AB system, 4H); MS: m/z 304 [M+], 197, 167, 149, 70, 57.
3. Results and discussion
Organic and inorganic reactions in H2O below 100 °C form an important part of chemical reactions. Kinetics and mechanisms of these reactions are the areas which are widely studied in chemistry. In high temperature water (e.g. below about 300 °C), preparative organic syntheses have been investigated recently, and a large number of examples are presented in this regard Citation5–9; however, few research works have been reported on the use of NCW and SCW (e.g. above 350 °C) for such “organic synthetic reactions” Citation10, Citation11, although SCW has been used mainly for “breakdown” of organic reactants such as destruction of waste and toxic organic compounds Citation12–14. Therefore, the present work has been undertaken to explore the further possibility of performing organic synthesis in SCW.
The most important factor for the high rates obtained in SCW may be the change in the nature of hydrogen bonding of H2O. Recent neutron diffraction data demonstrate that the hydrogen bonding is still present in SCW Citation15; however, the cooperative nature of the hydrogen bonding network disappears, and only dimers and monomers are predominant species Citation16–18. Large fluctuations of the structure near the critical point might allow further breakdown of monomers, leading to the evolution of protons from SCW medium itself Citation19–21. If the proton cannot escape, the local proton concentration would be high and might have a significant influence on reactivities in SCW region, especially in the near-critical region. Hence, we expect that acid-catalyzed organic syntheses can proceed under a SCW atmosphere even in the absence of any acid catalysts. In this paper, we first conduct a noncatalytic condensation of aniline with 4-chlorobenzaldehyde in NCW and SCW, which is well-known to be catalyzed by strong acids in conventional solutions Citation1. These reactions successfully afforded the desired product (3a) in high yield. Then a series of experiments was performed to optimize the model reaction conditions.
shows the temperature dependence of the yield of product. As shown in , the yield of product increased with increasing temperature, especially near the critical temperature, while the yield decreased markedly above the critical temperature. Therefore, the temperature of 350 °C was chosen for all further reactions.
Table 1. Yield of 3a at different temperatures after 3 min.
The best reaction time has been found to be 3 min at 350 °C and a further increase in time did not lead to a substantial improvement in yield.
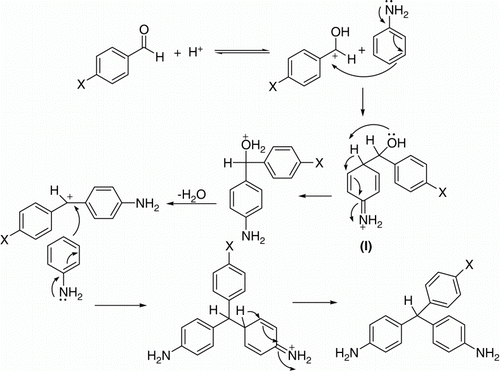
In near critical conditions (e.g. 350 °C), increased density has been shown to increase the product yields of products (). Considering the experimental results, a general mechanism for the condensation reaction in SCW is postulated as . The condensation reactions proceed through two steps: the first step is the equilibrium between aldehyde and protons which leads to the formation of carbocation on carbonyl group in aqueous solution. With increasing water density, this equilibrium probably shifts to the carbonium ion side through the contribution of solvating power for ionic species and proton concentration Citation22. The carbonium ion undergoes electrophilic attack with 1 mol of aniline and forms an intermediate (I). The second step of the reaction is the dehydration of intermediate, thereby producing the carbonium ion. The carbonium ion makes further electrophilic attack with another mol of aniline, which leads to the formation of diamino triphenyl methane.
Table 2. Yield of 3a at different water densities.
The effect of various substituents on the aromatic aldehydes on the reaction yield were tested at the optimized condition with aniline (). The aromatic aldehyde with electron-withdrawing groups showed higher reactivity than the electron-donating group.
Table 3. Condensation of aromatic aldehydes with aniline in near critical water.
Several catalysts have been reported for the condensation of aniline with aromatic aldehydes which include aqueous mineral acids as a homogeneous catalyst such as dry HCl Citation23, perfluorinated sulfonic acid resin and H3PO4 [1]. These catalysts have their own disadvantages and drawbacks. They generate many problems such as pollution, care of handling, safety, corrosion, and tedious workup procedures. Strong solid acids are suitable for replacement of liquid acids to decrease these disadvantages Citation24.
Heteropolyacids (HPAs) as supported or in bulk form, are very interesting solid acid catalysts, can act as green and ecofriendly catalysts. Being stronger acids, they generally exhibit higher catalyst activities than conventional catalysts such as mineral acids, ion exchange resins, mixed oxides, zeolites, etc. Citation25.
Recently, Ajaikumar et al. Citation1 reported that the bulk heteropoly acids and supported heteropoly acids showed better catalytic activity than the other materials for the synthesis of the industrially important diamino triphenyl methanes.
compares our results (time, yield, and reaction conditions) with results obtained by Ajaikumar et al. Citation1 for the synthesis of 3b. As can be seen, our method is simpler, more efficient, and uses no toxic solvents or catalyst.
Table 4. Comparison of our results with results obtained by other groups with some heterogeneous acid catalyst for the synthesis of 3b.
In a comparison with of the same reaction with other paper under non-conventional energy source (i.e. microwave), using aniline hydrochloride as catalyst in solvent-free conditions is also included, in this paper the synthesis of 3b was carried out in the same time (3 min) and with very similar yield (91%) but for other derivatives the yields were lower (67–90%) in this conditions Citation4.
4. Conclusion
The present results demonstrate that high-temperature water itself shows its real ability as an acid for remarkably accelerating reactions. This research has delivered a promising alternative approach compared to conventional methods and there is no need for environmentally less benign organic solvents or any additional catalyst or reagents.
Acknowledgements
The authors are thankful from Nuclear Science and Technology Research Institute for the partial financial support.
References
- Ajaikumar , S. and Pandurangan , A. 2008 . HPW and supported HPW catalyzed condensation of aromatic aldehydes with aniline: Synthesis of DATPM derivatives . J. Mol. Catal. A: Chem. , 286 : 21 – 30 .
- Ikushima , Y. , Hatakeda , K. , Sato , O. , Yokoyama , T. and Arai , M. 2000 . Acceleration of synthetic organic reactions using supercritical water: Noncatalytic Beckmann and Pinacol rearrangements . J. Am. Chem. Soc. , 122 : 1908 – 1918 .
- Fraga-Dubreuil , J. and Poliakoff , M. 2006 . Organic reactions in high-temperature and supercritical water . Pure Appl. Chem. , 78 : 1971 – 1982 .
- Guzmán-Lucero , D. , Guzmán , J. , Likhatchev , D. and MartÍnez-Palou , R. 2005 . Microwave-assisted synthesis of 4,40-diaminotriphenylmethanes . Tetrahedron Lett. , 46 : 1119 – 1122 .
- Katritzky , A.R. , Allin , S.M. and Siskin , M. 1996 . Aquathermolysis: reactions of organic compounds with superheated water . Acc. Chem. Res. , 29 : 399 – 406 .
- Kuhlmann , B. , Arnett , E.W. and Siskin , M. 1994 . Classical organic reactions in pure superheated water . J. Org. Chem. , 59 : 3098 – 3101 .
- Katritzky , A.R. ; Balasubramanian , M. ; Siskin , M. Dramatic effects caused by alkali metal salts on hydrolytic reaction rates of diaryl ethers in aqueous solutions at high temperatures (250 and 315 °C) . J. Chem. Soc., Chem. Commun . 1992 , 1233 – 1234 , doi: 10.1039/C39920001233 .
- Katritzky , A.R. , Lapucha , A.R. , Murugan , R. , Luxem , F.J. , Siskin , M. and Brons , G. 1990 . Aqueous high-temperature chemistry of carbo- and heterocycles. 1. Introduction and reaction of 3-pyridylmethanol, pyridine-3-carboxaldehyde, and pyridine-3-carboxylic acid . Energy & Fuels , 4 : 493 – 498 .
- An , J. , Bagnell , L. , Cablewski , T. , Strauss , C.R. and Trainor , R.W. 1997 . Applications of high-temperature aqueous media for synthetic organic reactions . J. Org. Chem. , 62 : 2505 – 2511 .
- Savage , P.E. 1999 . Organic chemical reactions in supercritical water . Chem. Rev. , 99 : 603 – 622 .
- Sato , O. , Ikushima , Y. and Yokoyama , T. 1998 . Noncatalytic beckmann rearrangement of cyclohexanone-oxime in supercritical water . J. Org. Chem. , 63 : 9100 – 9102 .
- Hatakeda , K. ; Ikushima , Y. ; Ito , S. ; Saito , N. ; Sato , O. Supercritical water oxidation of a PCB of 3-chlorobiphenyl using hydrogen peroxide . Chem. Lett . 1997 , 245 – 246 .
- Lee , D.-S. and Gloyna , E.F. 1990 . Efficiency of H2O2 and O2 in supercritical water oxidation of 2,4-dichlorophenol and acetic acid . J. Supercrit. Fluids. , 3 : 249 – 255 .
- Yang , H.H. and Eckert , C.A. 1988 . Homogeneous catalysis in the oxidation of p-chlorophenol in supercritical water . Ind. Eng. Chem. Res. , 27 : 2009 – 2014 .
- Bellissent-Funel , M.C. , Tassaing , T. , Zhao , H. , Beysens , D. , Guillot , B. and Guissani , Y. 1997 . The structure of supercritical heavy water as studied by neutron diffraction . J. Chem. Phys. , 107 : 2942 – 2950 .
- Hoffmann , M.M. and Conradi , S. 1997 . Are there hydrogen bonds in supercritical water? . J. Am. Chem. Soc. , 119 : 3811 – 3817 .
- Matsubayashi , M. , Wakui , C. and Nakahara , M. 1997 . NMR study of water structure in super- and subcritical conditions . Phys. Rev. Lett. , 78 : 2573 – 2576 .
- Matsubayashi , M. , Wakui , C. and Nakahara , M. 1997 . Structural study of supercritical water. I. Nuclear magnetic resonance spectroscopy . J. Chem. Phys. , 107 : 9133 – 9141 .
- Gorbaty , Y.E. and Kalinichev , A.G. 1995 . The isotropic to nematic liquid crystal transition for flexible nonspherical molecules . J. Chem. Phys. , 99 : 5336 – 5345 .
- Gorbaty , Y.E. and Demianets , Y.N. 1983 . The pair-correl.Ation functions of water at a pressure of 1000 bar in the temperature range 25–500°C . Chem. Phys. Lett. , 100 : 450 – 454 .
- Okhulkov , A.V. , Demianets , Y.N. and Gorbaty , Y.E. 1994 . X-ray scattering in liquid water at pressures of up to 7.7 kbar: Test of a fluctuation model . J. Chem. Phys. , 100 : 1578 – 1589 .
- Sato , T. , Sekiguchi , G. , Adschiri , T. , Smith , R.L. Jr and Arai , K. 2002 . Regioselectivity of phenol alkylation in supercritical water . Green Chem. , 4 : 449 – 451 .
- Hariharan , R. , Bhuvana , S. , Anuradha , G. and Sarojadevi , M. 2004 . Synthesis and characterisation of organosoluble polyimides containing the anisyl moiety . Polym. Int. , 53 : 1442 – 1447 .
- Heravi , M.M. , Ranjbar , L. , Derikvand , F. and Bamoharram , F.F. 2007 . H6P2W18O62: An efficient and reusable catalyst for one-pot synthesis of b-acetamido ketone and esters . Catal. Commun. , 8 : 289 – 291 .
- Hekmatshoar , R. , Heravi , M.M. , Sadjadi , S. , Oskooie , H.A. and Bamoharram , F.F. 2008 . Catalytic performance of Preyssler heteropolyacid, [NaP5W30O110]14– in liquid phase alkylation of phenol with 1-octene . Catal. Commun. , 9 : 837 – 841 .