Abstract
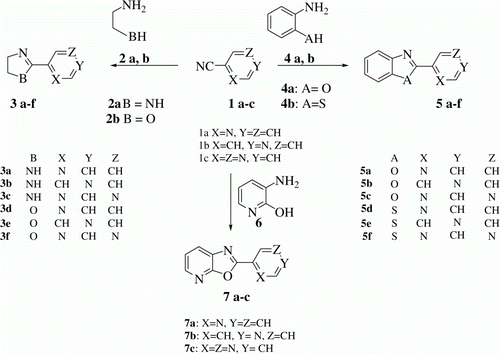
Dihydroimidazole (3a-c), dihydrooxazole (3d-f), benzoxazole (5a-c), benzothiazole (5d-f) and oxazolopyridine (7a-c) derivatives have been synthesized by condensation of various heterocyclic aromatic nitriles with diamine, aminoalcohal, aminophenol, aminothiophenol, and 3-aminopyridine-2-ol, respectively, under microwave irradiation and under solvent free conditions. This catalyst free and solvent free approach provided heterocyclic compounds in quantitative yields. Time taken for the condensation to occur is < 20 min.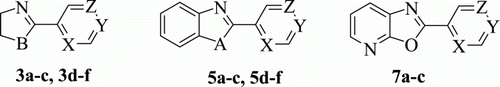
Introduction
Heterocyclic compounds bearing imidazole, oxazole, benzoxazole, benzothiazole, and oxazolopyridine moieties are of current research interest Citation1–6 due to their pharmacological applications Citation7. These types of heterocyclic compounds exhibit antitumor Citation8, Citation9 anti-inflammatory Citation10, Citation11, antibacterial Citation12, antiviral Citation13, and antimicrobial Citation14 activities. Since this investigation deals with a new approach to synthesize these medicinally important heterocyclic compounds, it is essential to review the procedures reported in literature. These procedures involve condensation via using (i) heterogeneous catalyst such as silphox with dimethylformamide Citation15, heteropolyacid supported on silica gel [Cu3/2PMo12O40/SiO2] Citation16, silica sulfuric acid Citation17, (ii) metal catalyst that is Pd(OAc)2PPh3 Citation18, H3PW12O40 Citation19, ZrOCl2.8H2O Citation20, CuCl & Cu(OTf)2 Citation21, (iii) solid support reagents Citation22, polyphosphoric acid Citation23, and N-bromosuccinimide Citation24. These heterocyclic molecules also have been synthesized by microwave irradiation Citation25–27, ultrasound irradiation by using catalyst Citation27, and by using ceric ammonium nitrate in presence of hydrogen peroxide under solvent free condition Citation28. Nitrile have also been converted to heterocyclic molecules by using catalyst and/or solvent under microwave irradiation Citation29–32. To the best of our knowledge no method is reported in literature involving condensation of heterocyclic aromatic nitriles with aliphatic diamino, aliphatic aminoalcohal, aromatic phenol, aromatic amino thiophenol, and hetroaromatic amino phenol using solvent free, catalyst free and microwave assisted conditions.
In related development microwave chemistry has a profound impact on the synthesis of heterocyclic compounds under solvent free condition, via using microwave irradiation for acceleration of organic reactions Citation33–36. Microwave irradiation technique reduces reaction time drastically and thus less chances of getting side products, which ultimately lead to high yields of required products and easy workup procedure.
In continuation of our efforts Citation37–40 in search of biologically important heterocyclic compounds which can be synthesized easily, we have synthesized dihydro-1H-imidazole, dihydrooxazole, benzoxazole, benzothiazole, and oxazolopyridine derivatives by reaction of heterocyclic aromatic nitriles with diamine, aminoalcohal, aminophenol, aminothiophenol, and 3-aminopyridine-2-ol, respectively, using microwave irradiation technique under solvent free condition, which we wish to report in this article.
Results and discussion
Equimolar ratio of 2-cyanopyridine 1a () and ethane 1,2 diamine 2a () were mixed together to make a homogeneous mixture and then this reaction mixture was subjected to microwave irradiation for 2 min. Thin layer chromatography (TLC) of reaction mixture showed formation of a new spot. This mixture was further subjected to microwave irradiation for 2×3 min. TLC of reaction mixture indicated absence of starting materials. This crude product was recrystallized from ethyl acetate to give pure product 3a in quantitative yield. IR, 1H NMR, GC-MS spectral and analytical data indicate that compound 3a is 2-(4,5-dihydro-1H-imidazol-2-yl)pyridine which is formed by addition of diamine on nitrile functional group and then elimination of one molecule of NH3. By following one step process heterocyclic molecules 3b-f were also synthesized in quantitative yield. Percentage yield and melting point (MP) of 3a-f are mentioned in .
Table 1. Physical constants of various heterocyclic compounds synthesized* i.e. 3a-f, 5a-f & 7a-c.
To further diversify this synthetic procedure we studied the reaction of 2-aminophenol 4a () and 2-cyanopyridine 1a () by irradiating their mixture in equimolar ratio for 1×5 min. A new product 5a was formed which was identified to be 2-(pyridin-2-yl)benzo[d]oxazole, which is formed by cyclization and loss of one molecule of NH3. By following one step process heterocyclic molecules 5b-f were also synthesized in quantitative yield. Percentage yield and MP of 5a-f are mentioned in .
To verify the versatility of our synthetic procedure we condensed 3-aminopyridin-2-ol (6; ) with 2-cyanopyridine, 4-cyanopyridine, and 2-cyanopyrazine (1a-c; ) under microwave irradiation and got heterocyclic cyclized product 7a-c in quantitative yield. Percentage yield and MP of 7a-c are mentioned in .
Experimental section
Microwave reactor model CEM Discover model No. 908010 were used for microwave irradiation. MP were determined on a JSGW apparatus and are uncorrected. IR spectra were recorded using a Perkin Elmer 1600 FT spectrometer. 1H NMR spectra were recorded on a Bruker WH-500 MHz NMR spectrometer at a ca 5–15% (w/v) solution in DMSO-d 6 (TMS as internal standard). GC-MS was recorded on Perkin Elmer Clarus 500 gas chromatograph where built in MS detector was used. Elemental analysis was carried out on a Vario EL III elementor. TLC was performed on silica gel G for TLC (Merck) and spots were visualized by iodine vapor or by irradiation with ultraviolet light (254 nm).
General procedure for synthesis of heterocyclic molecules
Reaction procedure for synthesis of 3a
Ethane-1,2-diamine (0.240 g, 4 mmol) and 2-cyanopyridine (0.416 g, 4 mmol) were mixed together thoroughly to form homogeneous reaction mixture. This reaction mixture was subjected to microwave irradiation for 2×4 min at 70°C. Completion of reaction was checked by TLC. Crude reaction product was crystallized from ethyl acetate to give pure product 3a. Similarly, compounds 3b-f, 5a-f, and 7a-c were prepared under microwaves. These reactions were also performed using household microwave oven (model M197DL, Samsung) and found comparable in terms of yield and reaction times.
2-(4,5-Dihydro-1H-imidazol-2-yl)-pyridine (3a)Citation41
Irradiation time 6 min. IR (KBr) νmax: 1639 (–C=N–), 1532 & 1461 (Ar) cm−1. 1H NMR (CDCl3) δ: 3.571 (bs, 2H, aliphatic), 4.09 (bs, 2H, aliphatic), 5.99 (bs, 1H, NH), 7.37–7.39 (m, 1H, py), 7.77–7.80 (m, 1H, py), 8.154–8.172 (dd, J 1=1 Hz, J 2=8 Hz, 1H, py), 8.592–8.60 (m, 1H, py). GC-MS m/z 147 (M+, 44.74%); Anal. Calcd. for C8H9N3 C: 65.30; H: 6.12; N: 28.57; Found C: 65.25; H: 6.08; N: 28.53%.
4-(4,-Dihydro-1H-imidazol-2-yl)-pyridine (3b)Citation41
Irradiation time 5 min. IR (KBr) νmax: 1630 (–C=N–), 1592 & 1545 (Ar) cm−1. 1H NMR (CDCl3) δ: 3.827–3.88 (bs, 4H, aliphatic), 4.851 (bs, 1H, NH), 7.646–7.658 (m, 2H, py), 8.713–8.725 (d, J 1=6 Hz, 2H, py). GC-MS m/z 147 (M+, 55.91%); Anal. Calcd. For C8H9N3 C: 65.30; H: 6.12; N: 28.57; Found C: 65.33; H: 6.10; N: 28.55%.
2-(4,5-Dihydro-1H-imidazol-2-yl)-pyrazine (3c)Citation42
Irradiation time 6 min. IR (KBr) νmax: 1601 (–C=N–), 1498 & 1461 (Ar), cm−1. 1H NMR (CDCl3) δ: 3.579 (bs, 2H, aliphatic), 4.040 (bs, 2H, aliphatic), 5.783 (s, 1H, NH), 8.466–8.474 (m, 1H, pyrazine), 8.573–8.578 (d, J 1=2.5 Hz, 1H, pyrazine), 9.309–9.311 (d, J 1=1 Hz, 1H, pyrazine). GC-MS m/z 148 (M+, 80.74%); Anal. Calcd. For C7H8N4 C: 56.75; H: 5.40; N: 37.83; Found C: 56.70; H: 5.42; N: 37.78%.
2-(4,5-Dihydro-oxazol-2-yl)-pyridine (3d)Citation43
Irradiation time 3 min. IR (KBr) νmax: 1658 (–C=N–), 1533 & 1469 (Ar), cm−1. 1H NMR (CDCl3) δ: 4.43–4.182 (t, J 1=9.5 Hz, 2H, aliphatic), 4.540–4.578 (t, J 1=9.5 Hz, 2H, aliphatic), 7.428–7.437 (m, 1H, py), 7.795–7.830 (m, 1H, py), 8.067–8.083 (d, J 1=8 Hz 1H, py), 8.730–8.739 (d, J 1=4.5 Hz 1H, py). GC-MS m/z 148 (M+, 26.75%); Anal. Calcd. For C8H8N2O C: 64.86; H: 5.40; N: 18.91; Found C: 64.83; H: 5.37; N: 18.90%.
4-(4,5-Dihydro-oxazol-2-yl)-pyridine (3e)Citation44
Irradiation time 4 min. IR (KBr) νmax: 1652 (–C=N–), 1547 & 1414 (Ar), cm−1. 1H NMR (CDCl3) δ: 4.116–4.155 (t, J 1=9.5 Hz, 2H, aliphatic), 4.489–4.528 (t, J 1=9.5 Hz, 2H, aliphatic), 7.803–7.815 (m, 2H, py), 8.737–8.749 (m, 2H, py). GC-MS m/z 148 (M+, 50.31%); Anal. Calcd. For C8H8N2O C: 64.86; H: 5.40; N: 18.91; Found C: 64.86; H: 5.35; N: 18.93%.
2-(4,5-Dihydro-oxazol-2-yl)-Pyrazine (3f)
Irradiation time 2 min. IR (KBr) νmax: 1653(–C=N–), 1573 & 1469 (Ar), cm−1. 1H NMR (CDCl3) δ: 4.168–4.207 (t, J 1=9.5 Hz, 2H, aliphatic), 4.547–4.585 (t, J 1=9.5 Hz, 2H, aliphatic), 8.682–8.692 (d, J 1=5 Hz, 2H, pyrazine), 9.277 (s, 1H, pyrazine). GC-MS m/z 149 (M+, 72.19%); Anal. Calcd. For C7H7N3O C: 56.37; H: 4.69; N: 28.18; Found C: 56.35; H: 4.65; N: 28.13%.
2-Pyridin-2-yl-benzooxazole (5a) Citation45
Irradiation time 5 min. IR (KBr) νmax: 1621 (–C=N–), 1591 & 1454 (Ar), cm−1. 1H NMR (DMSO-d 6) δ: 7.456–7.527 (m, 2H, Ar), 7.641–7.666 (m, 1H, py), 7.858–7.901 (m, 2H, Ar), 8.064–8.099 (dt, 1H, py), 8.351–8.371 (dt, 1H, py), 8.807–8.819 (m, 1H, py). GC-MS m/z 196 (M+, 100%); Anal. Calcd. For C12H8N2O C: 73.46; H: 4.08; N: 14.28; Found C: 73.40; H: 4.00; N: 14.25%.
2-Pyridin-4-yl-benzooxazole (5b) Citation23
Irradiation time 12 min. IR (KBr) νmax: 1633 (–C=N–), 1456 (Ar), Cm−1. 1H NMR (DMSO-d 6) δ: 7.470–7.551 (m, 2H, Ar), 7.866–7.918 (m, 2H, Ar), 8.115–8.127 (dd, J 1=1.5 Hz, J 2=4.5 Hz, 2H, py), 8.855–8.868 (d, J 1=2 Hz, J 2=4.5 Hz, 2H, py). Anal. Calcd. For C12H8N2O C: 73.46; H: 4.08; N: 14.28; Found C: 73.42; H: 4.02; N: 14.20%.
2-Pyrazin-2-yl-benzooxazole (5c)
Irradiation time 5 min. IR (KBr) νmax: 1684 (–C=N–), 1596 & 1446 (Ar), cm−1. 1H NMR (DMSO-d 6) δ: 7.494–7.572 (m, 2H, Ar), 7.901–7.938 (m, 2H, Ar), 8.891–8.906(d + s, 2H, pyrazine), 9.525–9.527 (d, J 1=1 Hz, 1H, pyrazine). GC-MS m/z 197 (M+, 100%); Anal. Calcd. For C11H7N3O C: 67.00; H: 3.55; N: 21.31; Found C: 66.94; H: 3.53; N: 21.35%.
2-Pyridin-2-yl-benzothiazole (5d) Citation46
Irradiation time 1 min. IR (KBr) νmax: 1643 (–C=N–), 1569 & 1506 (Ar) cm−1. 1H NMR (DMSO-d 6) δ: 7.495–7.528(m, 1H, Ar), 7.565–7.623 (m, 2H, Ar), 8.038–8.073 (m, 1H, Ar), 8.109–8.125 (d, J 1=8 Hz, 1H, py), 8.175–8.192 (m, 1H, py), 8.335–8.355 (m, 1H, py), 8.736–8.751 (m, 1H, py). GC-MS m/z 212 (M+, 100%); Anal. Calcd. For C12H8N2S C: 67.92; H: 3.77; N: 13.20; S: 15.09; found C: 67.90; H: 3.70; N: 13.24; S: 15.05%.
2-Pyridin-4-yl-benzothiazole (5e) Citation46
Irradiation time 2 min. IR (KBr) νmax: 1594 & 1469 (Ar), cm−1. 1H NMR (DMSO-d 6) δ: 7.560–7.642 (m, 2H, Ar), 8.044–8.056 (d, J 1=6 Hz, 2H, py), 8.241–8.257 (d, J 1=8 Hz, 2H, Ar), 8.803–8.815 (d, J 1=6 Hz, 2H, py). GC-MS m/z 212 (M+, 100%); Anal. Calcd. For C12H8N2S C: 67.92; H: 3.77; N: 13.20; S: 15.09; Found C: 67.89; H: 3.75; N: 13.15; S: 15.02%.
2-Pyrazin-2-yl-benzothiazole (5f) Citation47
Irradiation time 2 min. IR (KBr) νmax: 1630 (–C=N–), 1508 & 1447 (Ar) cm−1. 1H NMR (DMSO-d 6) δ: 7.533–7.632 (m, 2H, Ar), 8.161–8.177 (d, J 1=8 Hz, 1H, Ar), 8.217–8.233 (d, J 1=8 Hz, 1H, Ar), 8.820 – 8.828 (m, 1H, pyrazine), 8.849–8.854 (d, J 1=2.5 Hz, 1H, pyrazine), 9.509 (s, 1H, pyrazine). GC-MS m/z 213 (M+, 100%); Anal. Calcd. For C11H7N3S C: 61.97; H: 3.28; N: 19.71; S: 15.02; Found C: 61.94; H: 3.25; N: 19.67; S: 15.00%.
2-Pyridine-2-yl-oxazolo[4,5-b]pyridine (7a) Citation48
Irradiation time 4 min. IR (KBr) νmax: 1614 (–C=N–), 1584 & 1549 (Ar) cm−1. 1H NMR (DMSO-d 6) δ: 7.532–7.558 (m, 1H, Ar), 7.688–7.715 (m, 1H, Ar), 7.715– 8.100 (m, 1H, Ar), 8.325–8.344 (dd, J 1=1.5 Hz, J 2=8 Hz, 1H, py), 8.413–8.432 (m, 1H, py), 8.617–8.629 (dd, J 1=1 Hz, J 2=4.5 Hz, 1H, py), 8.840–8.849 (m, 1H, py). GC-MS m/z 197 (M+, 67.44%); Anal. Calcd. For C11H7N3O C: 67.00; H: 3.55; N: 21.31; Found C: 66.96; H: 3.49: N: 21.30%.
2-Pyridine-4-yl-oxazolo[4,5-b]pyridine (7b) Citation48
Irradiation time 20 min. IR (KBr) νmax: 1632 (–C=N–), 1586 & 1500 (Ar) cm−1. 1H NMR (DMSO-d 6) δ: 7.562–7.588 (q, 1H, Ar), 8.166–8.178 (dd, J 1=2 Hz, J 2=4.5 Hz, 2H, py), 8.350–8.369 (dd, J 1=1 Hz, J 2=8 Hz, 1H, Ar), 8.639–8.651 (dd, J 1=1 Hz, J 2=4.5 Hz, 1H, Ar), 8.900–8.912 (dd, J 1=1.5 Hz, J 2= 4.5 Hz, 2H, py). GC-MS m/z 197 (M+, 65.51%); Anal. Calcd. For C11H7N3O. C: 67.00; H: 3.55; N: 21.31; Found C: 63.25; H: 3.50; N: 21.25%.
2-Pyrazine-2-yl-oxazolo[4,5-b]pyridine (7c)
Irradiation time 8 min. IR (KBr) νmax: 1612 (–C=N–), 1551 & 1458 (Ar) cm−1. 1H NMR (DMSO-d 6) δ: 7.579–7.605 (q, 1H, Ar), 8.379–8.398 (dd, J 1=1 Hz, J 2=8 Hz, 1H, Ar), 8.662–8.674 (dd, J 1=1 Hz, J 2=4.5 Hz, 1H, Ar), 8.944 (s, 2H, pyrazine), 9.580–9.582 (d, J 1=1 Hz, 1H, pyrazine). GC-MS m/z 198 (M+, 68%); Anal. Calcd. For C10H6N4O. C: 60.60; H: 3.03; N: 14.14; Found C: 57.25; H: 2.50; N: 14.12%.
Conclusion
In conclusion, we have developed a very simple, high yielding, easy to workup one step process for conversion of heterocyclic aromatic nitriles to various heterocyclic compounds. Further efforts to synthesize more complex heterocyclic compounds are in progress.
Acknowledgements
We are thankful to the technical staff of Chemistry Department, I.I.T. Roorkee, for spectroscopic studies and elemental analysis and to Mr. Rakesh Kumar (Integral Biosciences Ltd. Noida) for helping to use microwave reactor. Ms. Surbhi Arya and Ms. Reshma Rani are thankful to CSIR, New Delhi, for financial assistance.
References
- Kumar , D. ; Rudrawar , S. ; Chakarborti , A.K. Aust. J. Chem . 2008 , 61 , 881 – 887 .
- Chakarborti , A.K. ; Rudrawar , S. ; Kirtikumar , B.J. ; Gurmeet , K. ; Sunay , V.C. Green Chemistry , 2007 , 9 , 1335 – 1340 .
- Motiwala , H.F. ; Kumar , J. ; Chakarborti , A.K. Aust. J. Chem . 2007 , 60 , 369 – 374 .
- Rudrawar , S. ; Kondaskar , A. ; Chakarborti , A.K. Synthesis 2005 , 15 , 2521 – 2526 .
- Parikh , N. ; Kumar , D. ; Roy , S.R. ; Chakarborti , A.K. Chem. Commun . 2011 , 47 , 1797 – 1799 .
- Chakarborti , A.K. ; Selvam , C. ; Kaur , G. ; Bhagat , S. Synlett . 2004 , 15 , 851 – 855 .
- Sawyer , T.K. In Topics in Medicinal Chemistry ; Bradbury , R.H. ; Berlin Springer-Verlag , 2007 , 1 , ch. 8 , pp 383 – 405 .
- Kamal , A. ; Reddy , K.S. ; Khan , M.N.A. ; Shetti , R.V.C.R.N.C. ; Ramaiah , M.J. ; Pushpavalli , S.N.C.V.L. ; Srinivas , C. ; Pal-Bhadra , M. ; Chourasia , M. ; Sastry , G.N. ; Juvekar , A. ; Zingde , S. ; Barkume , M. Bioorg. Med.Chem . 2010 , 18 , 4747 – 4761 .
- Sondhi , S.M. ; Rani , R. ; Partha , R. ; Agrawal , S.K. ; Saxena , A.K. Bioorg. Med.Chem. Lett . 2010 , 20 , 2306 – 2310 .
- Grice , C.A. ; Tays , K.L. ; Savall , B.M. ; Wei , J. ; Butler , C.R. ; Axe , F.U. ; Bembenek , S.D. ; Fourie , A.M. ; Dunford , P.J. ; Lundeen , K. ; Coles , F. ; Xue , X. ; Riley , J.P. ; Williams , K.N. ; Karlsson , L. ; Edwards , J.P. J. Med. Chem . 2008 , 51 , 4150 – 4169 .
- Khanum , S.A. ; Khanum , N.F. ; Shashikanth , M. Bioorg. Med. Chem. Lett . 2008 , 18 , 4597 – 4601 .
- Keurulainen , L. ; Salin , O. ; Siiskonen , A. ; Kern , J.M. ; Alvesalo , J. ; Kiuru , P. ; Maass , M. ; Yli-Kauhaluoma , J. ; Vuorela , P. J. Med. Chem . 2005 , 53 , 7664 – 7674 .
- Niedermeier , S. ; Singethan , K. ; Rohrer , S.G. ; Matz , M. ; Kossner , M. ; Diederich , S. ; Maisner , A. ; Schmitz , J. ; Hiltensperger , G. ; Baumann , K. ; Holzgrabe , U. ; Schneider-Schaulies , J. J. Med. Chem . 2009 , 52 , 4257 – 4265 .
- Jeyaprakash , R.S. ; Tiwari , M. ; Hashif , K. ; Srinivasan , K.K. ; Pharmacology online . 2009 , 3 , 737 – 742 .
- Hasaninejad , A. ; Niknam , K. ; Zare , A. ; Farsimadan , E. ; Shekouhy , M. Phosphorus, Sulfur Silicon Relat. Elem . 2009 , 184 , 147 – 155 .
- Fazaeli , R. ; Aliyan , H. Appl. Catal., A 2009 , 353 , 74 – 79 .
- Mohammadpoor-Baltork , I. ; Mirkhani , V. ; Moghadam , M. ; Tangestaninejad , S. ; Zolfigol , M.A. ; Abdollahi-Alibeik , M. ; Khosropour , A.R. ; Kargar , H. ; Hojati , S.F. Catal. Commun . 2008 , 9 , 894 – 901 .
- Zhuravlev , F.A. Tetrahedron Lett. , 2006 , 47 , 2929 – 2932 .
- Mohammadpoor-Baltork , I. ; Moghadam , M. ; Tangestaninejad , S. ; Mirkhani , V. ; Hojati , S.F. Catal. Commun . 2008 , 9 , 1153 – 1161 .
- Mohammadpoor-Baltork , I. ; Khosropour , A.R. ; Hojati , S.F. Catal. Commun . 2007 , 8 , 200 – 204 .
- Barbero , N. ; Carril , M. ; SanMartin , R. ; Dominguez , E. Tetrahedron 2007 , 63 , 10425 – 10432 .
- Radi , M. ; Saletti , S. ; Botta , M. Tetrahedron Lett . 2008 , 49 , 4464 – 4466 .
- Gao , R. ; Xiao , L. ; Hao , X. ; Sun , W.-H. ; Wang , F. Dalton Trans . 2008 , 5645 – 5651 .
- Fujioka , H. ; Murai , K. ; Ohba , Y. ; Hiramatsu , A. ; Kita , Y. Tetrahedron Lett . 2005 , 64 , 2197 – 2199 .
- Pottorf , R.S. ; Chadha , N.K. ; Katkevics , M. ; Ozola , V. ; Suna , E. ; Ghane , H. ; Regberg , T. ; Player , M.R. Tetrahedron Lett . 2003 , 44 , 175 – 178 .
- Saberi , A. ; Rangappa , K.S. Synth. React. Inorg. Me . 2009 , 39 , 425 – 427 .
- Moghadam , M. ; Mohammadpoor-Baltork , I. ; Mirkhani , V. ; Tangestaninejad , S. ; Abdollahi-Alibeik , M. ; Yousefi , B.H. ; Kargar , H. Monatsh. Chem . 2007 , 138 , 579 – 583 .
- Bahrami , K. ; Khodaei , M.M. ; Naali , F. J. Org. Chem . 2008 , 73 , 6835 – 6837 .
- Horneff , T. ; Chuprakov , S. ; Chernyak , N. ; Gevorgyan , V. ; Fokin , V.V. J. Am. Chem. Soc . 2008 , 130 , 14972 – 14974 .
- Sluiter , J. ; Christoffers , J. Synlett . 2009 , 63 – 66 .
- Chakarborti , A.K. ; Kaur , G. ; Roy , S. Indian J. Chem . 2001 , 40B , 1000 – 1006 .
- Chakarborti , A.K. ; Kaur , G. Tetrahedron . 1999 , 55 , 13265 – 13268 .
- Sondhi , S.M. ; Rani , R. Green Chem. lett. Rev . 2010 , 3 , 115 – 120 .
- Sondhi , S.M. ; Rani , R. ; Partha , R. ; Agrawal , S.K. ; Saxena , A.K. Bioorg. Med. Chem. Lett . 2009 , 19 , 1534 – 1538 .
- Kumar , R. ; Selvam , C. ; Kaur , G. ; Chakarborti ; A.K. Synlett . 2005 , 1402 – 1404 .
- Motiwala , H.F. ; Kumar , R. ; Chakarborti , A.K. Aust. J. Chem . 2007 , 1402 – 1404 .
- Sondhi , S.M. ; Dinodia , M. ; Jain , S. ; Kumar , A. Eur. J. Med. Chem . 2008 , 43 , 2824 – 2830 .
- Sondhi , S.M. ; Rani , R. Lett. Org. Chem . 2008 , 5 , 51 – 54 .
- Sondhi , S.M. ; Singh , J. ; Rani , R. ; Gupta , P.P. ; Agrawal , S.K. ; Saxena , A.K. Eur. J. Med. Chem . 2010 , 45 , 555 – 563 .
- Sondhi , S.M. ; Rani , R. ; Roy , P. ; Agrawal , S.K. ; Saxena , A.K. Eur. J. Med. Chem . 2010 , 45 , 902 – 908 .
- Nasr-Esfahani , M. ; Montazerozohori , M. ; Moghadam , M. ; Akhlaghia , P. ARKIVOC , 2010 , ii , 97 – 109 .
- Anastassiadou , M. ; Danoun , S. ; Crane , L. ; Baziard-Mouysset , G. ; Payard , M. ; Caignard , D.-H. ; Rettori , M.-C. ; Renard , P. Bioorg. Med. Chem . 2001 , 9 , 585 – 592 .
- Ishihara , M. ; Togo , H. Tetrahedron 2007 , 63 , 1474 – 1480 .
- Shaabani , A. ; Seyyedhamzeh , M. ; Maleki , A. ; Rezazadeh . F. Applied Catalysis A: General 2009 , 358 , 146 – 149 .
- Barbero , N. ; Carril , M. ; SanMartin , R. Dominguez , E. Tetrahedron 2007 , 63 , 10425 – 10432 .
- Deligeorgiev , T.G. Dyes Pigm . 1990 , 12 , 243 – 248 .
- Hassan , M.A. ; Zayed , S.E. ; El-Gaziri , W.N. ; Metwally , S.A. Arch. Pharm. (Weinheim) 1991 , 324 , 185 – 187 .
- Shen , T.-Y. ; Clark , R.L. ; Pessolano , A.A. ; Witzel , B.E. ; Lanza , T.J. U.S. 1977 , US 4038396 19770726 .