Abstract
A clean and operationally simple method has been developed for the preparation of mutual prodrug using paracetamol and various nonsteroidal anti-inflammatory drugs. The methodology involves use of TFAA/H3PO4 in acetonitrile and a variety of mutual prodrugs has been prepared in good yields by using this single-step C–O bond forming reaction.
Keywords:
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most commonly prescribed medicines across the world to treat pain Citation1. While they relieve aches, pains and are used to reduce joint inflammation seen with arthritis, they do increase the risk of stomach ulcers and gastrointestinal (GI) bleeding. About 1–4% of NSAID users experience GI complications Citation2. The direct contact mechanism is thought to play a determinant role in the formation of GI lesions. It is probably attributed to the free carboxylic group of the NSAIDs and to the local inhibition of the cytoprotective action of prostaglandins on gastric mucosa. Cyclooxygenase-2 (COX-2) inhibitors, a non ulcerogenic class of NSAIDs were developed to reduce GI side effects Citation3 Citation4. In the late 1990s two COX-2 inhibitors, Vioxx and Celebrex became two of the most prescribed medicines. After reports of Vioxx-related cardiovascular complications Citation5 the medicine was withdrawn from the market Citation6. The American Gastroenterology Association now recommends care to be taken prior to the decision to prescribe NSAIDs specifically, a careful balance to be achieved between the benefits of the medicine and its potential cardiovascular and GI complications Citation7. The association recommends stratifying patients into high or low risk for GI bleeding. Those with a previous history of peptic ulcer or Helicobacter pylori infection, history of NSAID-related gastric irritation, and the use of a combination of NSAIDs should be screened and treated for Helicobacter pylori and received an agent that protects the stomach's lining. It was also recommended that patients who are at greater risk of GI bleeding should receive NSAIDs with lower GI risks, such as COX-2 inhibitors, if their cardiovascular risk is minimal. Patients with a greater risk of cardiovascular problems should avoid COX-2 inhibitors and receive low-dose aspirin which is known to be cardioprotective. These new recommendations may allow significant reduction in the GI and cardiovascular complications that are associated with NSAIDs.
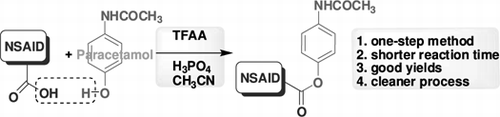
The aniline analgesic paracetamol is better tolerated at therapeutic doses in patients for whom excessive gastric acid secretion or prolongation of bleeding time may be a concern Citation8. But large overdoses of paracetamol can produce hepatotoxicity and nephrotoxicity. Thus, there is a great emphasis on research to discover methods aimed at improving their therapeutic efficacy by minimizing or eliminating these undesirable side effects. Sometimes, an adequate pharmaceutical formulation can overcome these drawbacks, but often the galenic formulation is ineffective and a chemical modification of active molecule is necessary to correct its pharmacokinetic insufficiencies. This chemical formulation process, whose objective is to convert a pharmacologically active molecule into a clinically acceptable drug, often involves the so-called “prodrug design.” Mutual prodrug is a type of carrier-linked prodrug, where the carrier used is another drug instead of some inert molecule Citation9. A mutual prodrug consists of two pharmacologically active agents coupled together so that each can act as a promoiety for the other agent and vice versa. It is an important area of research, and its introduction in human therapy has given successful results in improving the clinical and therapeutic effectiveness of drugs suffering from some undesirable effects that otherwise hinder their clinical applications. Thus, a number of mutual ester prodrugs of some of the NSAIDs have been prepared by using paracetamol as a masking agent for free carboxylic acid to reduce the ulcerogenic side effects of NSAIDs Citation10. Among the several methods Citation10–15 used for the esterification of NSAIDs the simplest and straightforward one being the esterification involving the use of corresponding acyl chlorides Citation10 Citation15 which are usually moisture sensitive and unstable. Most of the other methods used are also not environmentally friendly. For protection of the ecology, the reaction with lower environmental impact is therefore highly desirable. Herein we report trifluoroacetic anhydride (TFAA) and 85% H3PO4 mediated simple, mild and single step synthesis of mutual prodrugs (3) involving paracetamol (1) and a number of well known NSAIDs (2) ().
Results and discussion
We have previously reported that the neat reaction of phenol with carboxylic acid in presence of TFAA and 85% phosphoric acid at room temp or 50–60°C proceeds well leading to the formation of C–O bond thereby the corresponding ester Citation16. This handy methodology is being utilized elegantly for the preparation of various target compounds by other research groups Citation17. However, to our surprise under these conditions when employed, paracetamol and NSAIDs were found to be inert as no formation of desired ester was detected. Heating the reaction mixture to reflux also did not transform paracetamol and NSAIDs into the corresponding esters. We then examined the use of a solvent in this esterification reaction. Thus paracetamol (1) was reacted with aspirin (acetylsalicylic acid, 2a) in the presence of TFAA and 85% H3PO4 at room temperature in a number of solvents. The results of this study are summarized in . The use of benzene, chloroform and acetone was found to be less effective in terms of product yield (entries 1–3, ). The use of acetonitrile, however, improved the product yield significantly with dramatic decrease in reaction time (entry 4, ). Since increase in reaction time did not increase the product yield (entry 5, ) the reaction was then performed at higher temperature i.e. 60°C (entry 6, ). Indeed, the desired product 3a was isolated in 82% yield. Thus, acetonitrile was identified as best solvent for the preparation of our predesigned mutual prodrugs.
Table 1. Effect of solvents on the reaction of paracetamol (1) and aspirin (2a).a
We then used these optimized reaction conditions to prepare a variety of mutual prodrugs (). A comparison of reaction time and product yields for both the process i.e. the reaction carried out at room temperature and in acetonitrile at 60°C is also presented. NSAIDs other than aspirin (2a) such as indomethacin (2b), naproxen (2c) and ibuprofen (2d) reacted well with 1 to give the expected esters in good yields (entries 2–4, ). As observed earlier the use of acetonitrile was found to be advantageous in these cases too in compared to the neat reactions.
Table 2. Preparation of ester (3) via the reaction of paracetamol (1) with NSAIDs (2).a
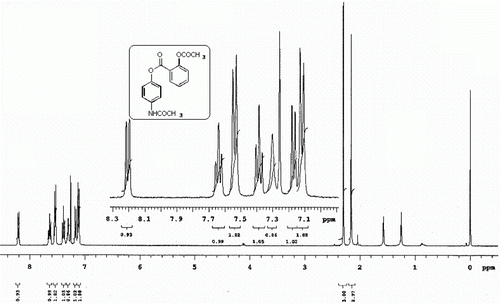
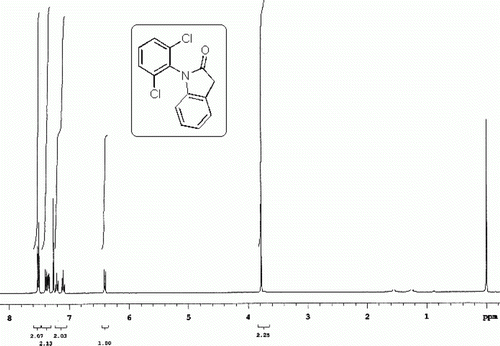
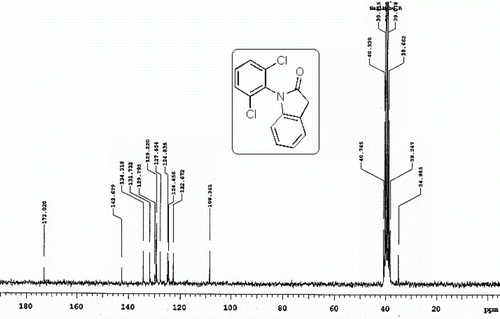
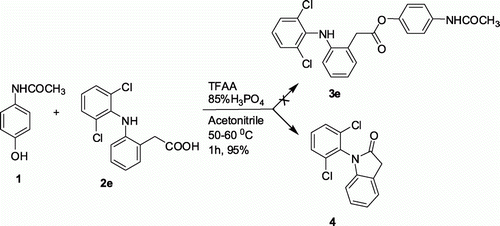
All the esters synthesized are confirmed by spectral data. The 1H NMR spectra of a representative compound 3a is shown in . Notably the use of dichlofenec (2e) as a NSAID in the present TFAA-H3PO4 mediated esterification reaction with 1 was found to be ineffective as a different compound other than the desired ester 3e was isolated in this case (). This compound later identified as 1-(2,6-dichlorophenyl)indolin-2-one (4) was thought to be generated as a result of intramolecular cyclodehydration reaction of 2e. The 1H and 13C NMR spectra of compound 4 ( and ) clearly indicate the absence of aromatic hydrogens due to the paracetamol moiety. On the other hand, the presence of a methylene group was indicated by the signal at~3.8 ppm and 34.9 ppm in the 1H and 13C NMR spectra, respectively. The best and perhaps the only method known for the preparation of compound 3e involve the use of chloroformate under the conditions as shown in Citation10.
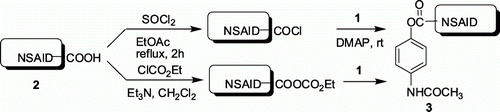
Traditionally, the mutual prodrugs can be synthesized via a two-step process Citation10 Citation15 i.e. acid chloride generation in situ followed by addition of paracetamol and catalytic amount DMAP and Et3N (). Alternatively, these compounds can be prepared via generating mixed anhydride using ethyl chloroformate in the presence of Et3N followed by reaction with paracetamol (). In an effort to understand the practical advantages of our method over these conventional methods we carried out a comparative study the results of which are summarized in . It is evident from that the present TFAA-H3PO4 mediated method provides comparable yields of products within a shorter period of time compared with the conventional methods (entries 1–4, ). Moreover, NSAID 2e provided compound 4 exclusively via TFAA-H3PO4 mediated method instead of a mixture of desired ester 3e and compound 4 via acid chloride method (entry 5, ).
Table 3. A comparison between the TFAA-H3PO4 mediated esterification of 1 and 2 with conventional two-step methods.
Notably, in-vitro and in-vivo evaluation of some of the mutual ester prodrugs was carried out earlier Citation10. Experimental data suggests that these esters possess adequate chemical stability, reduced ulcerogenic liability, enhanced lipophilicity, and improved bioavailability compared with the corresponding physical mixtures and the parent drugs. Thus, the mutual prodrugs synthesized by present TFAA/H3PO4 – mediated single-step method have potential medicinal value.
Experimental
General procedure for the synthesis of 3a-e
TFAA-H3PO4 mediated method
To a cold solution of NSAID (2, 1.0 mmol) and 85% H3PO4 (1.0 mmol), TFAA (4.0 mmol) in acetonitrile (5 mL) was added paracetamol (1, 1.0 mmol) and the mixture was stirred vigorously at 60°C for the time mentioned in . After completion of the reaction the crude mixture was added into crushed ice (20–30 g). The solid separated was filtered, washed with cold water (5 mL), collected and dried under vacuum. The solid isolated was purified by recrystallization from an appropriate single or a mixture of solvents to furnish the desired product.
Acid chloride method
A mixture of NSAID (0.01 mol) and thionyl chloride (5 mL) in ethylacetate (10 mL) was allowed to reflux for 2 h. Excess of thionyl chloride and ethyl acetate was removed by distillation under reduced pressure. The resulting acyl chloride formed was dissolved in dry CH2Cl2 and added dropwise to a cold and stirred mixture of paracetamol (0.01 mmol), triethylamine (0.01 mol) and DMAP (0.001 mol) in CH2Cl2 (15 mL). The reaction mixture was stirred at room temperature for the time indicated in . After completion of the reaction the mixture was concentrated under low vacuum and added to crushed ice (100 g). The solid separated was filtered, dried, and recrystallized to furnish the desired product.
4-Acetamidophenyl 2-acetoxybenzoate (3a)
Off white solid (recrystallized from ethyl acetate); mp. 183–185°C; Rf=0.24 (ethyl acetate/chloroform 2:8); MS m/z 314 (M+100%); IR vmax/cm–1 (KBr): 3322, 1770, 1745, 1666; 1H-NMR (CDCl3 400 MHz) δ 8.20 (1H, d, J=7.8 Hz), 7.65 (1H, ArH, t, J=7.7 Hz), 7.54 (2H, ArH, d, J=9.0 Hz), 7.38 (1H, ArH, t, J=7.6 Hz), 7.32 (1H, NH, bs), 7.18 (1H, ArH, d, J=8.2 Hz), 7.10 (2H, ArH, d, J=9.0 Hz), 2.56 (3H, Me, s) 2.16 (3H, Me, s); 13C-NMR (DMSO-d 6 , 100 MHz) δ 169.2 (CO), 168.3 (CO), 162.9 (CO), 150.3 (C), 145.3 (C), 137.3 (C), 135.0 (C), 131.7 (CH), 126.5 (CH), 124.1 (CH), 122.3 (CH), 121.8 (CH), 119.9 (CH), 23.9 (CH3), 20.7 (CH3); Elemental Analysis C 65.45, H 4.67 C17H15NO5 requires C 65.17, H 4.83, N 4.47.
Acetamidophenyl 2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetate (3b)
White solid (recrystallized from ethyl acetate); mp 187–189°C; Rf=0.24 (ethyl acetate/chloroform 2:8); MS m/z 491 (M + 100%); IR vmax/cm–1 (KBr):3327, 1730, 1670, 1617; 1H-NMR: (CDCl3 400 MHz) δ 7.62 (2H, ArH, d, J = 3 Hz), 7.45 (4H, ArH, m), 7.18 (1H, NH, s, D2O exchangeable), 7.00 (3H, ArH, m), 6.90 (1H, ArH, m), 6.85 (1H, ArH, m), 3.83 (2H, CH2, s), 3.8 (3H, Me, s), 2.50 (3H, Me, s), 2.20 (3H, Me, s); 13C NMR (DMSO-d 6 , 100 MHz) δ 174.8, 170.2, 169.5, 155.6, 137.7, 136.9, 135.6, 134.1, 131.2, 130.8, 130.1, 129.1, 121.7, 119.8, 114.7, 112.3, 111.2, 101.6, 55.4, 29.3, 23.9, 13.3.
4-Acetamidophenyl-2-(6-methoxynaphthalen-2-yl)propanoate (3c)
Off white solid (recrystallized from ethanol); mp 178–176°C; Rf=0.28 (ethyl acetate/chloroform 2:8); MS m/z 364 (M + 100%); IR vmax/cm–1 (KBr): 3296, 1738, 1669; 1H-NMR (DMSO-d 6 , 300 MHz) δ 7.73 (2H, m), 7.49–7.43 (2H, m), 7.16 (NH, bs), 7.15 (5H, m), 6.93 (1H, d, J=6.7 Hz), 4.07 (1H, q, J=7.0 Hz), 3.90 (3H, s), 2.14, (3H, s), 1.67 (3H, d, J=7.1 Hz).
4-Acetamidophenyl 2-(4-isobutylphenyl)propanoate (3d)
White solid; mp 86–88°C; Rf=0.42 (ethyl acetate/chloroform 2:8); MS m/z 340 (M + 100%); IR vmax/cm–1 (KBr): 3379, 1720, 1684; 1H-NMR: (DMSO-d 6 , 300 MHz) δ 7.44 (2H, d, J=8.3 Hz), 7.28 (2H, d, J=8.3 Hz), 7.20 (NH, s, D2O exchangeable), 7.13 (2H, ArH, d, J=7.9 Hz), 6.93 (2H, ArH, d, J=8.6 Hz), 3.92 (q, 1H, J=7.0 Hz), 2.46 (d, 2H, J=7.0 Hz), 2.19 (3H, s), 1.80 (1H, m), 1.58 (d, 3H, J=6.9 Hz), 1.57 (d, 6H, J=6.9 Hz).
4-Acetamidophenyl 2-(2-(2, 6-dichlorophenylamino)phenyl)acetate (3e)
White solid; mp 188–190°C Rf=0.62 (ethyl acetate/hexane 2:3); MS m/z 429 (M + 100%); IR vmax/cm–1 (KBr): 3192, 3113, 2987, 1685; 1H-NMR (DMSO-d 6 , 300 MHz) δ 6.5–7.5 (11H, m, phenyl + NH) 7.2 (2H, d, J =7 Hz, H ortho to -OCO-), 6.8 (2H, d, J=8 Hz, H ortho to -NHCO), 4.0 (2H, s, -CH2COO), 2.1 (3H, s, NHCOCH3).
1-(2,6-Dichlorophenyl)indolin-2-one (4)
Buff colored solid; mp 121–123°C (lit Citation18 121–123°C); Rf=0.71 (chloroform).
Conclusion
In conclusion, a direct and efficient method has been developed for the preparation of mutual prodrug using paracetamol and various NSAIDs. The combination of TFAA/H3PO4 has been identified as an effective coupling agent for this purpose. The use of acetonitrile was found to be critical without which the reaction did not proceed. A variety of mutual prodrugs has been prepared by using this methodology in good yields. Our study indicated that the present one-step method is more effective than the conventional methods reported earlier. Because of the medicinal value of the compounds synthesized the present operationally simple and clean esterification process would find wide usage especially in the preparation of other prodrugs.
Acknowledgements
The authors (K. K. and S. P.) thank Mr M. N. Raju, the chairman of MNR Educational Trust for constant support and encouragement.
References
- Inotai , A. ; Hanko , B. ; Meszaro , A. Pharmacoepidemiol. Drug Saf . 2010 , 19 , 183 – 190 .
- Pezzilli , R. ; Morselli-Labate , A.M. ; Corinaldesi , R. Pharmaceuticals 2010 , 3 , 558 – 571 .
- Talley , J.J. Prog. Med. Chem. Res . 1999 , 36 , 201 – 234 .
- Black , W.C. Annu. Rep. Med. Chem . 2004 , 39 , 125 – 128 .
- Mukherjee , D. ; Nissen , S.E. ; Topol , E.J. JAMA . 2001 , 286 , 954 – 959 .
- Warde , N. Nat. Rev. Rheumatol . 2011 , 7 , 129 .
- NSAIDs side effects. http://www.insidermedicine.com/archives/NSAIDs_Side_Effects_98.aspx (accessed July 11, 2011) .
- Graham , G.G. ; Scott , K.F. ; Day , R.O. Drugs . 2003 , 63 , 43 – 46 .
- Bhosle , D. ; Bharambe , S. ; Gairola , N. ; Dhaneshwar , S.S. Indian J. Pharm. Sci . 2006 , 68 , 286 – 294 .
- Fadl , T.A. Omar , F.A. Inflammopharmacology 1998 , 6 , 143 – 157 .
- Spadaro , A. ; Bucolo , C. ; Ronsisvalle , G. ; Pappalardo , M. J. Pharm. Sci. & Res . 2009 , 1 , 88 – 95 .
- De Caprariis , P. ; Palagiano , F. ; Bonina , F. ; Montenegro , L. ; D'Amico , M. ; Rossi , F. J. Pharm. Sci . 1994 , 83 , 1578 – 1581 .
- Bandarage , U.K. ; Chen , L. ; Fang , X. ; Garvey , D.S. ; Glavin , A. ; Janero , D.R. ; Gordon Letts , L. ; Mercer , G.J. ; Saha , J.K. ; Schroeder , J.D. ; Shumway , M.J. ; Tam , S.W. J. Med. Chem . 2000 , 4 , 4005 – 4016 .
- Halen , P.K. ; Chagti , K.K. ; Giridhar , R. ; Yadav , M.R. Med. Chem. Res . 2007 , 16 , 101 – 111 .
- Wechter , W.J. ; Schwartz , E.B. ; World Patent Application No WO/2004/032845 .
- Kankanala , K. ; Reddy , V.R. ; Mukkanti , K. ; Pal , S. J. Fluorine Chem . 2009 , 130 , 505 – 508 .
- Bhatt , L.R. ; Kim , B.M. ; Hyun , K. ; Kang , K.H. ; Lu , C. ; Chai , K.Y. Carbohydrate Res . 2011 , 346 , 691 – 694 ; Bhatt, L.R.; Kim, B.M.; Hyun, K.; Kwak, G.B.; Lee, C.H.; Chai, K.Y. Molecules 2011, 16, 3029–3036 .
- Chung , M.C. ; dos Santos , J.L. ; Oliveira , E.V. ; Blau , L. ; Menegon , R.F. ; Peccinini , R.G. Molecules 2009 , 14 , 3187 – 3197 .
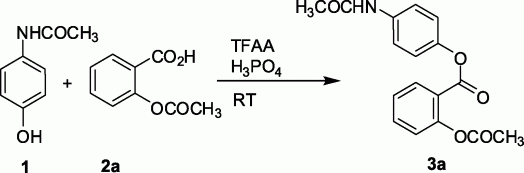
1H NMR (CDCl3) spectra of 3a
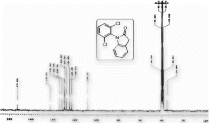
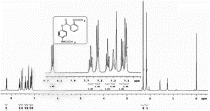 13C NMR (DMSO-d
6
) spectra of 3a
13C NMR (DMSO-d
6
) spectra of 3a
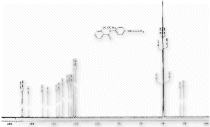
1H NMR (CDCl3) spectra of 3b
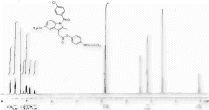 13C NMR (DMSO-d
6
) of 3b
13C NMR (DMSO-d
6
) of 3b
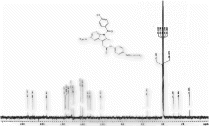
1H NMR (CDCl3) spectra of 3c
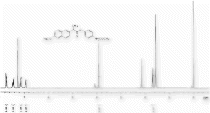 13C NMR (CDCl3) spectra of 3c
13C NMR (CDCl3) spectra of 3c
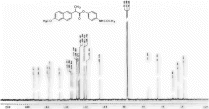
1H NMR (CDCl3) spectra of 3d
 13C NMR (CDCl3) spectra of 3d
13C NMR (CDCl3) spectra of 3d
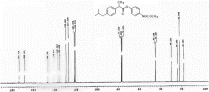
1H NMR (CDCl3) spectra of 4.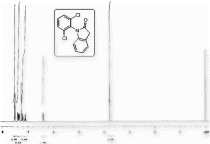 13C NMR (DMSO-d6) spectra of 4.
13C NMR (DMSO-d6) spectra of 4.