Abstract
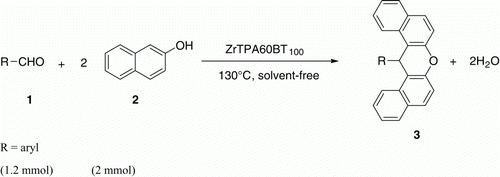
A simple and efficient procedure has been developed for the preparation of aryl-14H-dibenzo[a,j]xanthenes by a one-pot condensation of 2-naphthol and aryl aldehydes, in the presence of mesoporous zirconia modified with tungstophosphoric acid (ZrTPA60BT100), to be used as a heterogeneous catalyst in a solvent-free medium using conventional heating. The present approach offers the advantages of clean reaction, simple methodology, short reaction time, and high yield. The reaction work-up is very simple and the catalyst can be easily separated from the reaction mixture and reused several times in subsequent reactions without appreciable loss of the catalytic activity.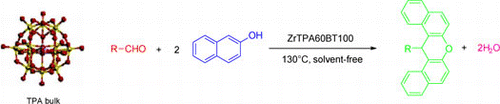
1. Introduction
The preparation of organic compounds involving a greener process and under solvent-free conditions has been investigated worldwide due to stringent environmental and economic regulations. The implementation of several transformations in a single manipulation is highly compatible with the goals of Green Chemistry Citation1–3.
In the last two decades, inorganic solid-catalyzed organic transformations have gained much importance due to the proven advantage of heterogeneous catalysis, such as simplified product isolation, mild reaction conditions, high selectivities, easy catalyst recovery and reuse, and reduction in generation of waste byproducts Citation4–6.
Particularly, the catalysis by heteropolyacids (HPAs) and related compounds is a field of increasing importance worldwide. Numerous developments are being carried out in basic research as well as in fine chemistry processes Citation7. Due to the strong acidic properties of solid HPAs, in the last decades, HPAs have found applications as useful and versatile acid catalysts for some acid-catalyzed reactions Citation8 Citation9. They can be used in bulk or supported form in both homogeneous and heterogeneous systems. Furthermore, HPAs have several advantages, including high flexibility in modification of acid strength, environmental compatibility, nontoxicity, and ease of handling Citation10.
On the other hand, zirconia is an interesting material to be used as catalyst support due to its thermal stability in different atmospheres. Its acid properties can be modified by the addition of cationic or anionic substances, such as sulfate or tungstate Citation11–13. The addition of Keggin HPAs to modify them has been studied to a lesser extent Citation14–17.
The most common methods that can be used to obtain zirconia are the sol-gel method, the micellar technique, or mechanochemical synthesis Citation16–18. Zirconia has been frequently prepared by the micellar method, while the sol-gel method from an alkoxide has been less used Citation16 Citation18.
On the other hand, xanthenes and benzoxanthene derivatives have received considerable interest from the pharmaceutical industry due to their wide range of interesting biological and therapeutic properties Citation19 such as anti-inflammatory Citation20, antibacterial Citation21, antiviral Citation22, CNS stimulating Citation23 activity as well as their use in photodynamic therapy (PDT: a method of treating tumors by the combined use of photosensitizer and light) Citation24 and for antagonism of the paralyzing action of zoxazolamine Citation25. The other useful applications of these heterocycles are as dyes Citation26, fluorescent materials for visualization of biomolecules Citation27, luminescent sensors Citation28, and in laser technologies Citation29.
One of the best reported protocols for the synthesis of 14-aryl-14H-dibenzo-[a,j]xanthenes involves the mixing of 2-naphthol with aldehydes in the presence of a catalyst Citation30, such as AcOH-H2SO4 Citation31, p-TSA Citation32, iodine Citation33, Amberlist-15 Citation34, P2O5/Al2O3 Citation35, silica sulfuric acid Citation36, Dowex-50W Citation37, ionic liquid Citation38, bulk Citation39, and silica-supported Citation40 HPAs.
As reported by Kumar and co-workers Citation41, some of these methods show varying degrees of success as well as limitations such as long reaction times, low yields, use of toxic solvents, and harsh reaction conditions. Thus, the development of an alternative clean procedure is highly needed for the synthesis of benzoxanthenes and relative compounds. In this direction our research group has experience in the friendly synthesis of heterocycle compounds using HPAs, for example, coumarins Citation42, dihydropyrimidinones Citation43, azlactones Citation44, and flavones Citation45.
As part of our research project directed towards the development of highly suitable methods and the synthesis of diverse heterocyclic compounds, we herein disclose a new, one-pot synthesis of 14-aryl-14H-dibenzo[a,j]-xanthenes from aldehydes and 2-naphthol catalyzed by mesoporous zirconia modified with tungstophosphoric acid under solvent-free conditions with the aim to obtain high yields with an environmentally advantageous procedure ().
2. Results and discussion
In continuation of our interest in the application of heteropolyacid catalysts for the development of a useful synthetic methodology, we wish to report a simple and efficient approach for the preparation of 14-aryl-14H-dibenzo[a,j]xanthenes using mesoporous zirconia modified with tungstophosphoric acid as an eco-friendly catalyst with high catalytic activity under solvent-free conditions at 100 °C.
Initially we conducted blank experiments without the presence of a catalyst. In order to obtain the optimal reaction temperature, four temperatures (80, 100, 120 and 130 °C) were tested. The experimental reaction conditions were: 1.2 mmol of benzaldehyde, 2 mmol of 2-naphtol, solvent-free conditions, and a reaction time of 1.5 h. No reaction was observed at 80 and 100 °C (, entries 1 and 2). A temperature increase gives low substrate conversion. The yield of 14-phenyl-14H-dibenzo[a,j]xanthene for a reaction time of 1.5 h at 120 °C was only 15% (, entry 3), whereas at 130 °C the yield was 32% (, entry 4). It was also observed that for a reaction time of 5 h, the yield decreased 2% when the reaction temperature was 130 °C (, entry 5).
Table 1. Reaction between 2-naphthol and benzaldehyde under different conditions.
Afterwards, the catalytic activity of the bulk H3PW12O40 (TPA) was tested in the preparation of 1-phenyl-14H-dibenzo[a,j]xanthene. The experimental reaction conditions were 1.2 mmol of benzaldehyde, 2 mmol of 2-naphthol, 50 mg of bulk catalyst, and solvent-free conditions. No reaction was observed at 80 °C and only traces were obtained at 100 °C for a reaction time of 1 h (, entries 6 and 7, respectively). The yields of 1-phenyl-14H-dibenzo[a,j] xanthene increased notably for a reaction time of 1 h at 130 °C. In this case the yield was 90% (, entry 9).
Finally, we studied the catalytic activity of the mesoporous zirconia modified with tungstophosphoric acid prepared in the present work. The ZrTPA60BT100 was tested in the preparation of 1-phenyl-14H-dibenzo[a,j]xanthene. The experimental reaction conditions were 1.2 mmol of benzaldehyde, 2 mmol of 2-naphthol, 85 mg of supported catalyst, and solvent-free conditions. No reaction was observed at 80 °C and only a 10% yield of product was obtained at 100 °C for a reaction time of 3 h (, entries 10 and 11). The yield of 1-phenyl-14H-dibenzo[a,j]xanthene increased notably for a reaction time of 1 h at 130 °C. In this case, the yield was 99% (, entry 13), being noticeably more active than the bulk catalyst.
The reusability of the catalyst was checked by separating the mesoporous zirconia modified with tungstophosphoric acid catalyst from the reaction mixture by simple filtration, washing with toluene, and drying in a vacuum oven at room temperature for 4 h prior to reuse in subsequent reactions without significant loss in product yields (, entries 14, 15, and 16).
After optimization of the reaction conditions, we studied the generality of this condition to other substrates. Using this method, different substrates were reacted with 2-naphthol to produce the corresponding 14-aryl-14H-dibenzo[a,j]xanthenes at 130 °C in solvent-free conditions. The results are summarized in . Several aromatic aldehydes with different functional groups were subjected to the condensation reaction, and the desired products were synthesized in good to excellent yields. The substituted functional groups in the aromatic ring of the aldehyde affect the yield and the reaction time. In comparison with electron-withdrawing groups in the aldehyde, we found that the presence of electron-donating groups in the aldehyde decreased both the reaction rate and the yields of products (, entries 8, 9, and 10).
Table 2. Synthesis of aryl-14H-dibenzo[a,j]xanthenes with ZrTPA60BT100 as catalyst.a
3. Experimental
All chemical reagents and solvents were obtained from Aldrich and were used without further purification. All products are known compounds and were identified by comparison of their physical and spectral data with those of the authentic samples. Melting points were measured on a Bioamerican Bs 448 apparatus and were uncorrected. 1H-NMR and 13C-NMR spectra were recorded on a Varian 200 MHz. The purity of the substances and the progress of the reaction were monitored by thin layer chromatography (TLC) on silica gel, and yields refer to isolated products.
3.1. Preparation of catalyst
Zirconium propoxide (Aldrich, 26.6 g) was mixed with absolute ethanol (Merck, 336.1 g) and stirred for 10 min to obtain a homogeneous solution under N2 at room temperature. Then 0.47 cm3 of 0.28 M HCl aqueous solution was dropped slowly into the aforementioned mixture to catalyze the sol-gel reaction.
After 3 h, an appropriate amount of PEG–alcohol–water (1:5:1 weight ratio) solution was added to the hydrolyzed solution under vigorous stirring to act as template. The amount of added solution was fixed in order to obtain a template concentration of 10% by weight in the final material. A TPA solution (whose concentration was fixed in order to obtain TPA concentration of 60% w/w in the solid) was added together with the addition of PEG (sample ZrTPA60B).
The gel was kept in a beaker at room temperature up to dryness. The solid was ground into powder and extracted with distilled water for three periods of 8 h, in a system with continuous stirring, to remove PEG. Afterwards, the solids were calcined at 100 °C for 24 h.
3.2. Typical procedure for aryl-14H-dibenzo[a,j] xanthenes synthesis using ZrTPA60BT100 catalyst
A mixture of the aldehyde (1.2 mmol), 2-naphthol (2 mmol), and ZrTPA60BT100 (85 mg) was stirred at 130 °C for the appropriate time according to . Completion of the reaction was indicated by TLC The reaction mixture was cooled to 25 °C, toluene (5 cm3) was added, and then the mixture was stirred for 15 min and filtered to separate the catalyst, which was subsequently washed twice with toluene (3 cm3). The combined toluene extracts were washed twice with water (5 cm3), dried over anhydrous Na2SO4 and evaporated in vacuo. The solid obtained was recrystallized from ethyl alcohol to afford the pure 14-aryl-14H-dibenzo[a,j]xanthenes derivatives. After the reaction, the catalyst was filtered, washed thoroughly with toluene, dried in a vacuum oven (room temperature, 4 h) and reused for the next reaction, following the procedure described earlier.
3.3. Representative spectral 1H-NMR and 13C-NMR data
14-(4-Methylphenyl)-14H-dibenzo[a,j]xanthenes (, entry 2): Mp: 237–239 °C. 1H-NMR (200 MHz, CDCl3): δ 2.15 (s, 3H), 6.37 (s, 1H), 6.92 (d, J=9.5 Hz, 2H), 7.30–7.78 (m, 12H), 8.33 (d, J=9.4Hz, 2H). 13C-NMR (50 MHz, CDCl3): δ 19.2, 35.2, 115.8, 116.3, 121.3, 122.8, 125.3, 126.5, 127.6, 129.3, 129.3, 129.4, 129.5, 134.0, 140.8, 146.6.
14-(4-Methoxylphenyl)-14H-dibenzo[a,j]xanthenes (, entry 3): 211–214 °C. 1H-NMR (200 MHz, CDCl3): δ 3.55 (s, 3H), 6.42 (s, 2H), 6.64 (d, J=9.6 Hz, 2H), 7.30–7.82 (m, 12H), 8.35 (d, J=9.6 Hz, 2H). 13C-NMR (50 MHz, CDCl3): δ 36.5, 53.1, 114.3, 117.4, 118.5, 123.6, 124.2, 127.3, 128.1, 129.2, 129.5, 131.5, 133.8, 137.4, 149.1, 158.1.
14-(4-Chlorophenyl)-14H-dibenzo[a,j]xanthenes (, entry 5): 288–290 °C. 1H-NMR (200 MHz, CDCl3): δ 6.44 (s, 1H), 7.08–8.32 (m, 16H). 13C-NMR (50 MHz, CDCl3): 37.4, 116.8, 118.2, 122.5, 125.4, 126.9, 128.7, 129.0, 129.5, 129.6, 131.1, 131.3, 132.0, 143.5, 148.7.
4. Conclusion
In conclusion, ZrTPA60BT100 as a noncorrosive, environmentally friendly and stable heterogeneous material has been proved to be a very efficient catalyst for the synthesis of dibenzoxanthene derivatives. A convenient and green process for the synthesis of aryl-14H-dibenzo[a,j]xanthenes by condensation of various aromatic aldehydes with 2-naphthol using ZrTPA60BT100 as a heterogeneous acid catalyst in a solvent-free medium using conventional heating at 130 °C has been developed. In addition, short reaction times, excellent yields, a straightforward procedure, and relative nontoxicity of the catalyst are other noteworthy advantages of the present method. Finally, this solid acid catalyst can be recovered and reused at least four times with negligible loss in its activity. Further applications of ZrTPA60BT100 in green organic transformations are currently in progress in our laboratory.
Acknowledgements
The authors thank Agencia Nacional de Promoción Científica y Tecnológica, CONICET, and Universidad Nacional de La Plata for financial support, and L. Soto, L. Osiglio, D. Peña, and N. Firpo for their experimental collaboration in the experimental measurements.
References
- Kumar , R. ; Nandi , G. ; Verma , R. ; Singh , M. Tetrahedron Lett . 2010 , 51 , 442 – 445 .
- Rahmati , A. Chinese Chem. Lett . 2010 , 21 , 761 – 764 .
- Kumar , R. ; Nandi , G. ; Verna , R. ; Singh , M. Tetrahedron Lett . 2010 , 51 , 442 – 445 .
- Clark , J. Acc. Chem. Res . 2002 , 35 , 791 – 797 .
- Okuhara , T. Chem. Rev . 2002 , 102 , 3641 – 3666
- Wilson , K. ; Clark , J.H. Pure Appl. Chem . 2000 , 72 , 1313 – 1319 .
- Misono , M. ; Nojiri , N. Appl. Catal. A: Gen . 1990 , 64 , 1 – 30 .
- Amini , M. ; Seyyedhamzeh , M. ; Bazgir , A. Appl. Catal. A: Gen . 2007 , 323 , 242 – 245 .
- Kozhevnikov , I. J. Mol. Catal. A: Chem . 2007 , 262 , 86 – 92 .
- Kozhevnikov , I. Chem. Rev . 1998 , 98 , 171 – 198 .
- Yadav , G. ; Nair , J. Micropor. Mesopor. Mater . 1999 , 33 , 1 – 48 .
- Boyse , R. ; Ko , E. J. Catal . 1997 , 171 , 191 – 207
- Zhao , B. ; Xu , X. ; Ma , H. ; Sun , D. ; Gao , J. Catal. Lett . 1997 , 45 , 237 – 244 .
- Devassy , B. ; Shanbhag , G. ; Halligudi , S. J. Mol. Catal. A: Chem . 2006 , 247 , 162 – 170
- Mallik , S. ; Dash , S. ; Parida , K. ; Mohapatra , B. J. Colloid Interf. Sci . 2006 , 300 , 237 – 243 .
- Qu , X. ; Guo , Y. ; Hu , Ch. J. Mol. Catal. A: Chem . 2007 , 262 , 128 – 135 .
- Pizzio , L. ; Vázquez , P. ; Cáceres , C. ; Blanco , M. Catal. Lett . 2001 , 77 , 233 – 239 .
- Fernández-García , M. ; Martínez-Arias , A. ; Hanson , J. ; Rodríguez , J. Chem. Rev . 2004 , 104 , 4063 – 4104 .
- Madhav , J. ; Reddy , Y. ; Reddy , P. ; Reddy , M. ; Kuarm , S. ; Crooks , P. ; Rajitha , B. J. Mol. Catal. A: Chem . 2009 , 304 , 85 – 87 .
- Chibale , K. ; Visser , M. ; Schalkwyk , D. ; Smith , P. ; Saravanamuthu , A. ; Fairlamb , A. Tetrahedron 2003 , 59 , 2289 – 2296
- El-Brashy , A. ; Metwally , M. ; El-Sepai , F. Farmaco 2004 , 59 , 809 – 817 .
- Jamison , J. ; Krabill , K. ; Hatwalkar , A. Cell. Biol. Int. Resp . 1990 , 14 , 1075 – 1084 .
- Lewis , J. ; Readhead , M. J. Med. Chem . 1970 , 13 , 525 .
- Ion , R. Prog. Catal . 1997 , 2 , 55 .
- Saint-Ruf , G. ; De , A. ; Hieu , H. Bull. Chim. Ther . 1972 , 7 , 83 – 86 .
- Banerjee , A. ; Mukherjee , A. Stain Technol . 1981 , 56 , 83 – 85 .
- Knight , C. ; Stephenes , T. Biochem. J . 1989 , 258 , 683 – 689 .
- Callan , J. ; De Silva , P. ; Magri , D. Tetrahedron 2005 , 61 , 8551 .
- Sirkecioglu , O. ; Tulinli , N. ; Akar , A. J. Chem. Res . ( S ) 1995 , 502 – 503 .
- Mirjalili , B. ; Bamoniri , A. ; Akbari , A. Chim. Chem. Lett . 2011 , 22 , 45 – 48 .
- Sharma , R. ; Baruah , J. Dyes Pigments 2005 , 64 , 91 .
- Khosropour , A. ; Khodaei , M. ; Moghannian , H. Synlett 2005 , 995 .
- Das , B. ; Ravikanth , B. ; Ramu , R. ; Laxminarayana , K. ; Rao , B. J. Mol. Catal. A: Chem . 2006 , 255 , 74 .
- Ko , S. ; Yao , C. Tetrahedron Lett . 2006 , 47 , 8827 .
- Zarei , A. ; Hajipour , A. ; Khazdooz , L. Dyes Pigments 2010 , 85 , 133 – 138 .
- Mozhdeh , S. ; Peiman , M. ; Bazgir , A. Dyes Pigments 2008 , 76 , 836
- Shakibaei , G. ; Mirzaei , P. ; Bazgir , A. Appl. Catal. A: Gen . 2007 , 325 , 188 – 192 .
- Kanteravi , S. ; Chary , M. ; Das , A. ; Vuppalapati , S. ; Lingaiah , N. Catal. Commun . 2008 , 9 , 1575 – 1578 .
- Nagarapu , L. ; Kantevari , S. ; Mahankhali , V. ; Apuri , S. Catal. Commun . 2007 , 8 , 1173 – 1177 .
- Amini , M. ; Seyyedhamzeh , M. ; Bazgir , A. Appl. Catal. A: Gen . 2007 , 323 , 242 – 245 .
- Kumar , R. ; Nandi , G. ; Verna , R. ; Singh , M. Tetrahedron Lett . 2010 , 51 , 442 – 445 .
- Romanelli , G. ; Bennardi , D. ; Ruiz , D. ; Baronetti , G. ; Thomas , H. ; Autino , J. Tetrahedron Lett . 2004 , 45 , 8935 – 8939 .
- Romanelli , G. ; Sathicq , A. ; Autino , J .; Thomas , H. ; Baronetti , G. Synth. Commun . 2007 , 37 ( 22 ), 3907 – 3916 .
- Romanelli , G. ; Autino , J. ; Vázquez , P. ; Pizzio , L. ; Blanco , M. ; Cáceres , C. Appl. Catal. A: Gen . 2009 , 352 , 208 – 213 .
- Bennardi , D. ; Romanelli , G. ; Autino . J. ; Pizzio , L. ; Vázquez , P. ; Cáceres , C .; Blanco , M. React. Kinet. Mechanism Catal . 2010 , 100 , 165 – 174 .