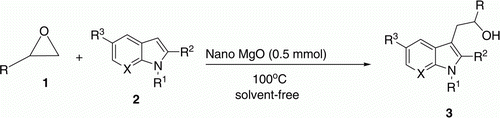
Abstract
3-Alkylindole derivatives were produced by ring opening of epoxides in the presence of magnesium oxide nanoparticles as an efficient catalyst. Solvent-free conditions, stoichiometric amounts of reactants, and improved reaction times and yields are the most important advantages of this method. MgO nanoparticles are synthesized successfully. X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and Brunauer- Emmet-Teller (BET) surface measurement were used to characterize the structure and morphology of the catalyst.
Introduction
As indole derivatives especially 3-alkylindoles have significant biological and medicinal importance Citation1–4, there are great efforts to obtain the best reaction conditions and highest yields in the synthesis of these compounds. Because of the nucleophilic nature of indole compounds, different methods have been used for their alkylation Citation5–15 . Friedel-Crafts alkylation of indoles with α,β-unsaturated N-acylbenzotriazoles Citation16 and with nitroalkenes Citation17 have been reported. However, epoxides are one of the most usable compounds in alkylation of indoles. Ring opening of epoxides with indoles has been carried out using Lewis acids such as RuCl3·nH2O Citation18, sulfated zirconia Citation19, SbCl3/K-10 Citation20, LiClO4 Citation21–24, Ln(OTf)3 Citation25, InCl3 Citation26, InBr3 Citation27, HBF4–SiO2 Citation28, and silicagel Citation29. Furthermore the ring opening of epoxides with indoles has been reported by using high pressure conditions Citation30 Citation31, or ionic liquid Citation32. Most of these methods suffer from some disadvantages such as large excess amount of reagents, high pressure, low yields, poor regioselectivity, and special efforts are needed to prepare the catalyst.
Due to their wide applications as catalyst and catalyst support Citation33, nanocrystalline metal oxides seem to be suitable candidates to catalyze the epoxide opening reaction with indoles. Recently, Kantam et al. reported Friedel-Crafts alkylation of indoles with nanocrystalline titanium oxide Citation34. However, the success of this method is limited to aromatic epoxides, and the method did not avoid the use of toxic-halogenated organic solvents Citation35 Citation36 such as dichloromethane and involved longer reaction times (10–62 h).
Magnesium oxide as a heterogeneous basic catalyst has been used for several organic transformations Citation37. It is widely used in toxic waste remediation and also in steel manufacturing because of its high resistance against corrosion Citation38. There are many reports in which MgO is characterized as an adsorbent Citation39–42. As surface structure is responsible for activity and selectivity of a material, it is desirable to achieve a high surface area of magnesium oxide Citation43. MgO nanoparticles show unusual surface reactivity. High surface area causes nanoscale MgO to be an efficient material in many processes such as dehalogenation and adsorption of various gases Citation44. Having all of the above in mind and in continuation of our interest in developing environmentally benign protocols via solvent-free conditions and using nanometal oxides as catalysts Citation45–52, we report herein our results with nano MgO that efficiently catalyzed Friedel-Crafts alkylation of indoles under solvent-free conditions ().
Results and discussion
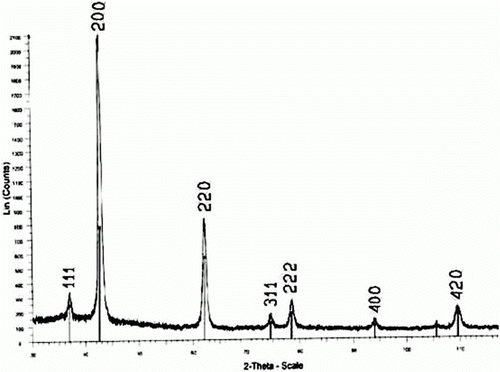
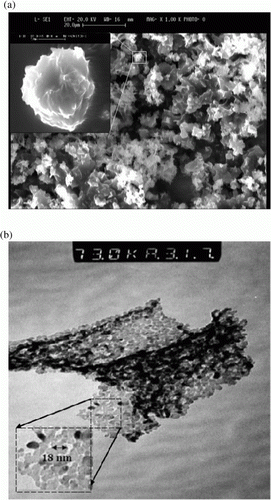
In this study, we prepared nano-sized MgO catalyst according to the procedure reported by Niu et al. Citation53. The XRD pattern of the synthesized MgO particles in the 2κ range of 30–110° is shown in . The diffraction angle and intensity of the characteristic peaks of the samples is consistent with that of the standard JCPDS card no. 4-0829. The value of 20 nm was calculated from XRD data for average particle size of this crystalline MgO using Scherrer's equation Citation34. The scanning electron micrograph (SEM) and transmission electron micrograph (TEM) of the synthesized MgO particles are shown in . It was observed that the synthesized product was nano MgO flake-like and the wall of the flake is about 18–30 nm thickness as shown in b, which is in good agreement with XRD crystal sizes. In addition, by low temperature N2 adsorption technique, the difference between the surface of synthesized nano MgO and commercially MgO was determined as 119 m2/g.
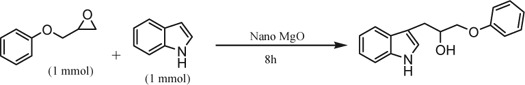
After synthesis and characterization of nano MgO, its catalytic activity was examined in Friedel-Crafts alkylation of indoles. To show the catalytic activity of nano MgO as a convenient and efficient catalyst, the reaction between glycidyl phenyl ether (1a) and indole (2a) was chosen as a model and its behavior was studied under a variety of conditions via GC and NMR spectroscopy (). A detailed literature survey towards the synthesis of 1-(1H-indol-3-yl)-3-phenoxypropan-2-ol (3a) from the reaction between glycidyl phenyl ether (1a) and indole (2a) revealed that a few papers have been published Citation12 Citation14 Citation19 . However these methods suffer from disadvantages. It was reported that when such reactions were carried out in refluxing acetonitrile in the presence of Yb(OTf)3 for 9 days only less than 20% of compound (3a) was obtained Citation25. This reaction could also be catalyzed by SiO2 Citation30 Citation31 under high pressure for 3 days and in the presence of SbCl3/montmorillonite K-10 Citation20 with poor yield (58%). To our delight, the corresponding product 1-(1H-indol-3-yl)-3-phenoxypropan-2-ol was obtained in 70% yield when the reaction was performed in the presence of nano MgO.
Table 1. Optimized Friedel-Crafts alkylation reaction of indole (1 mmol) with glycidyl phenyl ether (1 mmol) using nano MgO under various reaction conditions after 8h.
According to , the Friedel-Crafts alkylation of indole with glycidyl phenyl ether finished in 8 h at 100°C by using 0.5 mmol of the catalyst to give the corresponding product 1-(1H-indol-3-yl)-3-phenoxy-2-propanol in 70% yield. The lower yield and longer reaction time was observed at lower temperature (80°C) than the optimal reaction temperature (100°C) (entry 2). Control experiment in the absence of nano MgO at 100°C gave only a trace amount of product after 1 week. On the other hand, this catalyst was not efficient in the presence of organic solvents (entries 3–7). This observation confirms that the solvent-free conditions play an important role in this reaction. So, a rate enhancement was observed when the reaction was carried out without any solvent. Because of decreasing in the yields of the reactions in the presence of solvent, we suggested that nano MgO surface interacted with the solvents and this may be deactivated the catalyst Citation55. Unlike many previously reported methods, present protocol did not require excess amount of reactants and the mole ratio of epoxide:indole was 1:1. Further studies showed that the amount of nano MgO had influenced on the reaction yield in the optimum reaction time (8 h). The best amounts of nano MgO were 0.5 and 1 mmol, which afforded the desired product in 70% yields after 8 h. However, when the amount of nano MgO decreased, the yield of the product also decreased. So, less than 0.5 mmol, nano MgO was not an effective catalysis (entries 8–11).
To evaluate the scope of catalyst application, various structurally divergent epoxides were tested with substituted indoles under the optimized conditions and the results are shown in .
Table 2. Friedel-Crafts alkylation of indoles with epoxides using nano MgO under solvent-free condition at 100°C.
Aliphatic and aromatic epoxides reacted with indoles in the presence of nano MgO to give good yields of substitution products with high regioselectivity. The regioselectivity was determined by 1H NMR spectral data. It was evident that the reaction of aliphatic oxiranes occurred exclusively at the less hindered carbon of the epoxides ring, resulting in the formation of secondary alcohols (, entries 1–7). However, aryl epoxides underwent cleavage by indoles with preferential attack at the benzylic position, resulting in the formation of primary alcohols (, entries 8–12). In the case of 4-methyl indole 2e with a group located at the 4-position reacted with 1h smoothly to give regioselecively the corresponding alkylated indoles 3l (, entry 12). As the 3-position of indole is the preferred site for electrophilic substitution reactions, 3-alkyl indole derivatives were obtained exclusively in all reactions.
In order to show the accessibility of the present work in comparison with some other metal oxide catalysts reported previously or prepared in our laboratory, we summarized some of the results for the preparation of 1-(1H-indol-3-yl)-3-phenoxy-2-propanol (3a) in , which shows that nano MgO is the most efficient catalyst with respect to the reaction time and temperature and exhibits broad applicability in terms of yield.
Table 3. Friedel-Crafts alkylation of indole with glycidyl phenyl ether under various reported methods.a
Apart from the mild conditions and environmentally benign of the process and its excellent results, the simplicity of product isolation and the possibility to recover and recycle of nano MgO as catalyst offer significant advantages. The catalyst can be directly separated by a simple centrifuge from the reaction mixture after adding ethyl acetate. The catalyst after drying in an oven at 100°C can directly be reused. Studies using of indole with glycidyl phenyl ether as model substrates showed that the recovered nano MgO could be successively recycled without any decrease of yields ().
Table 4. Reuse ability of recovered nano MgO.a
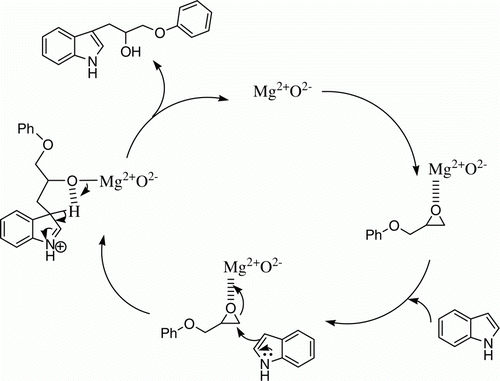
No attempt has been made to probe the mechanism of the reaction. However, proposed mechanism for the role of nano MgO is shown in . Nanocrystalline MgO contains large number of basic sites such as O2– and O–, and Mg2 + as Lewis acid sites. We proposed that indole undergoes nueophilic addition to epoxide at less hindered carbon where Lewis acid sites of MgO (Mg2 +) are coordinated to the oxygen of the epoxide, resulting in the increased of its reactivity.
Experimental
Except 2-((naphthalen-1-yloxy)methyl)oxirane (1f) and 2-(m-tolyloxymethyl)oxirane (1g) (, entries 6 and 7) which were prepared and purified by column chromatography in our laboratory, all chemicals were purchased from Fluka or Merck Chemical Companies. TLC Monitoring: silica gel Polygrams SIL G/UV 254 plates. 1H NMR and 13C NMR spectra were measured on Bruker Advance DPX FT 250 and 62.9 MHz spectrometry with TMS as an internal standard. Mass spectra were obtained on a Shimadzu GCMS0QP 1000EX at 20 and/or 70 eV. Power X-ray diffraction (XRD) was performed on a Bruker D8-advance X-ray diffractometer with Cu Ka (λ=1.54178 Å) radiation. The FT-IR spectra were recorded on an impact 400D Nickolet FT-IR spectrophotometer. The morphology of the products was determined by using Leica Cambridge, model s360, version V03.03 Scanning electron microscopy (SEM) performed at accelerating voltage of 25 Kv. The size was confirmed by Philips CM10 TEM instrument.
General procedure for the Friedel-Crafts alkylation of indole using non-nanometal oxides
All commercial metal oxides were purchased from Fluka or Merck Companies and their crystalline sizes and specific surface area was determined in our laboratory using XRD, TEM, and TG spectroscopy.
A mixture of glycidyl phenyl ether (1 mmol), indole (1 mmol), and metal oxides (commercial MgO, TiO2, ZnO, and nano ZnO) (0.5 mmol) were stirred at 100°C in an oil-bath under solvent-free condition. When the reaction was completed (monitored by TLC), the mixture was extracted with ethylacetate (three times). Then the product was purified by column chromatography.
General procedure for the Friedel-Crafts alkylation of indoles with epoxides using nano MgO
A mixture of epoxide (1 mmol), indole derivatives (1 mmol), and nano MgO (0.5 mmol, 0.02 g) were stirred at 100°C in an oil-bath under solvent-free condition. When the reaction was completed (monitored by TLC or GC chromatography), the mixture was extracted with ethylacetate (three times). Then the product was purified by column chromatography.
Spectral and analytical data for all compounds
1-(1H-indol-3-yl)-3-phenoxy-2-propanol (3a)
Purple solid; M.p 81–83°C (lit. 82–84oC) Citation18; IR (KBr) ? (cm–1): 3056, 3326, 3416, 3545; 1H NMR (CDCl3, 250 MHz) d 2.60 (s, 1H, –OH), 3.13 (m, 2H, Ar–CH 2 –), 3.98 (m, 2H, PhO–CH 2 –), 4.37 (m, 1H, –CH(OH)–), 6.91–7.35 (m, 9H, Ar–H), 7.67 (s, 1H, Ar–H), 8.22 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 29.39, 70.10, 71.10, 111.20, 114.58, 118.83, 119.60, 121.03, 122.22, 122.90, 127.14, 129.47, 136.51, 158.22; MS m/z (%): 57 (100.0), 83 (56.4), 111 (19.3), 129 (14.7), 148 (9.3), 167 (7.1), 189 (7.6), 236 (7.1), 255 (4.5), 267 (M+, 4.5), 268 (M++1, 1.4), 269 (M++2, 2.8).
1-Chloro-3-(1H-indol-3-yl)-2-propanol (3b)
Purple oil; IR (neat) ? (cm–1): 3370, 3450; 1H NMR (CDCl3, 250 MHz) d 2.24 (s, 1H, –OH), 3.07 (m, 2H, Ar–CH 2 –), 3.62 (m, 2H, –CH 2 –Cl), 4.17 (m, 1H, –CH(OH)–), 7.18 (m, 4H, Ar–H), 7.62 (s, 1H, Ar–H), 8.08 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 30.13, 49.24, 71.23, 110.92, 111.24, 118.76, 119.70, 122.34, 122.98, 127.53, 136.57; MS m/z (%): 57 (44.1), 81 (21.5), 97 (16.7), 130 (100.0), 149 (19.4), 209 (M+, 7.5), 210 (M++1, 0.5).
2-(1H-indol-3-yl)cyclohexanol (3c)
Purple solid; M.p 149–152°C (lit. 157°C) Citation56 Citation57; IR (KBr) ? (cm–1): 3050, 3355, 3460; 1H NMR (CDCl3, 250 MHz) d 1.41 (m, 4H, –CH 2 -cyclohexyl), 1.80 (m, 5H, –CH 2 -cyclohexyl, –OH), 2.77 (m, 1H, –CH(Ar)–), 3.77 (m, 1H, –CH(OH)–), 7.09–7.39 (m, 4H, Ar–H), 7.73 (d, 1H, Ar–H, J = 7.4 Hz), 8.11 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 23.81, 24.16, 29.77, 33.06, 67.04, 73.50, 110.92, 111.24, 118.76, 119.70, 122.34, 122.98, 127.53, 136.57; MS m/z (%): 57 (100.0), 83 (36.8), 130 (95.8), 149 (43.1), 215 (M+, 32.6), 216 (M++1, 7.6).
1-Bromo-3-(1H-indol-3-yl)-2-propanol (3d)
Purple oil; IR (neat) ? (cm–1): 3365, 3447; 1H NMR (CDCl3, 250 MHz) d 2.17 (s, 1H, –OH), 3.05 (m, 2H, Ar–CH 2 –), 3.55 (m, 2H, –CH 2 –Br), 4.09 (m, 1H, –CH(OH)–), 7.18 (m, 4H, Ar–H), 7.62 (s, 1H, Ar–H), 8.08 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 30.02, 46.59, 70.63, 110.89, 111.24, 118.76, 119.70, 122.34, 122.98, 127.53, 136.57; MS m/z (%): 57 (100.0), 83 (44.6), 99 (18.0), 130 (32.9), 149 (18.0), 167 (12.9), 184 (5.1), 255 (M++1, 3.9), 256 (M++2, 2.4).
1-(1H-indol-3-yl)-2-propanol (3e)
Dark pink solid; M.p 127–130°C (lit. 129–133°C) Citation54; IR (KBr) ? (cm–1): 3065, 3340, 3430; 1H NMR (CDCl3, 250 MHz) d 1.23 (m, 3H, –CH 3 ), 2.07 (s, 1H, –OH), 2.99 (m, 2H, Ar–CH 2 –), 3.75 (m, 1H, –CH(OH)–), 7.14 (m, 4H, Ar–H), 7.56 (s, 1H, Ar–H), 8.10 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 23.19, 42.65, 69.66, 110.89, 111.24, 118.76, 119.70, 122.34, 122.98, 127.53, 136.57; MS m/z = 175, calc. 175.
1-(1H-indol-3-yl)-3-(naphthalen-1-yloxy)-2-propanol (3f)
Purple solid; M.p 94–97°C; IR (KBr) ? (cm–1): 1038, 1245, 3052, 3320, 3416, 3545; 1H NMR (CDCl3, 250 MHz) d 2.47 (s, 1H, –OH), 3.24 (m, 2H, Ar–CH 2 –), 4.16 (m, 2H, ArO–CH 2 –), 4.50 (m, 1H, –CH(OH)–), 6.77 (s, 1H, Ar–H), 7.02–7.55 (m, 9H, Ar–H), 7.68 (s, 1H, Ar–H), 8.07 (s, 1H, Ar–H), 8.34 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 29.67, 70.29, 71.27, 105.05, 111.16, 111.34, 118.85, 119.62, 120.67, 121.04, 121.88, 122.21, 123.15, 125.41, 125.59, 125.93, 126.55, 127.64, 134.55, 136.34, 154.30; MS m/z (%): 69 (41.0), 97 (13.9), 130 (100.0), 317 (M+, 13.7), 318 (M++1, 4.7); Anal. Calc. for C21H19NO2: C, 79.49; H, 5.99; N, 4.41. Found: C, 79.45; H, 5.98; N, 4.63.
1-(1H-indol-3-yl)-3-(m-tolyloxy)-2-propanol (3g)
Purple oil; IR (neat) v (cm–1): 1035, 1234, 3067, 3345, 3450; 1H NMR (CDCl3, 250 MHz) d 2.31 (s, 3H, Ar–CH 3 ), 2.55 (br, 1H, –OH), 3.11 (m, 2H, Ar–CH 2 –), 3.99 (m, 2H, ArO–CH 2 –), 4.35 (m, 1H, –CH(OH)–), 6.78 (m, 3H, Ar–H), 7.16 (m, 4H, Ar–H), 7.35 (s, 1H, Ar–H), 7.65 (d, 1H, Ar–H, J = 7.3 Hz), 8.13 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 21.50, 29.41, 70.14, 71.13, 111.24, 111.35, 111.50, 115.49, 118.85, 119.56, 121.89, 122.18, 122.97, 127.58, 129.23, 136.32, 139.55; MS m/z (%): 69 (83.4), 95 (16.3), 130 (100.0), 149 (12.3), 281 (M+, 17.4), 282 (M++1, 6.7).
2-(1H-indol-3-yl)-2-phenylethanol (3h)
Purple solid; M.p 128–130°C (lit. 130–130.5°C) Citation34; IR (KBr) v (cm–1): 3088, 3330, 3431; 1H NMR (CDCl3, 250 MHz) d 2.55 (s, 1H, –OH), 4.01 (m, 2H, –CH 2 –OH), 4.31 (t, 1H, Ph–C(H)–, J = 6.6 Hz), 6.69 (s, 1H, Ar–H), 6.93–7.64 (m, 9H, Ar–H), 8.36 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 45.59, 66.39, 111.34, 115.73, 119.34, 119.45, 122.09, 122.18, 125.97, 126.73, 127.02, 128.35, 128.62, 136.50, 141.80, 142.29; MS m/z (%): 69 (62.6), 91 (15.7), 107 (10.4), 130 (25.2), 149 (23.9), 178 (24.8), 206 (100.0), 237 (M+, 12.6), 238 (M++1, 3.0).
2-(1-methyl-1H-indol-3-yl)-2-phenylethanol (3i)
Dark yellow oil; IR (neat) v (cm–1): 2970, 3080, 3385; 1H NMR (CDCl3, 250 MHz) d 2.55 (s, 1H, –OH), 3.41 (s, 3H, –N-CH 3 ), 4.09 (m, 2H, -CH 2 -OH), 4.31 (t, 1H, Ph–C(H)–, J = 6.6 Hz), 6.69 (s, 1H, Ar–H), 6.93–7.64 (m, 9H, Ar–H); 13C NMR (CDCl3, 62.9 MHz) d 39.75, 45.61, 66.39, 111.34, 115.73, 119.34, 119.45, 122.09, 122.18, 125.97, 126.73, 127.02, 128.35, 128.62, 136.50, 141.80, 142.29; MS m/z (%): 57 (21.3), 93 (23.5), 120 (20.0), 146 (45.7), 162 (19.3), 178 (12.6), 220 (100.0), 251 (M+, 14.8), 252 (M++1, 3.7).
2-(2-methyl-1H-indol-3-yl)-2-phenylethanol (3j)
Dark yellow oil; IR (neat) v (cm–1): 2980, 3050, 3370, 3455; 1H NMR (CDCl3, 250 MHz) d 2.22 (s, 3H, Ar–CH 3 ), 2.56 (s, 1H, –OH), 4.01 (m, 2H, –CH 2 –OH), 4.31 (t, 1H, Ph–C(H)–, J = 6.6 Hz), 6.90–7.74 (m, 9H, Ar–H), 8.16 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 21.05, 45.59, 66.39, 111.34, 115.73, 119.34, 119.45, 122.09, 125.97, 126.73, 127.02, 128.35, 128.62, 129.64, 136.50, 141.80, 142.29; MS m/z (%): 77 (13.5), 104 (10.8), 130 (17.5), 146 (25.1), 178 (11.1), 220 (100.0), 251 (M+, 20.1), 252 (M++1, 4.0), 253 (M++2, 1.1).
2-(5-Bromo-1H-indol-3-yl)-2-phenylethanol (3k)
Brown oil; IR (neat) v (cm–1): 3095, 3373, 3450; 1H NMR (CDCl3, 250 MHz) d 2.69 (s, 1H, –OH), 4.12 (m, 2H, –CH 2 –OH), 4.38 (t, 1H, Ph–C(H)–, J = 6.6 Hz), 6.72 (s, 1H, Ar–H), 7.21–7.75 (m, 8H, Ar–H), 8.39 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 45.59, 66.39, 111.34, 115.73, 118.51, 119.45, 122.09, 122.18, 126.22, 126.73, 127.14, 128.35, 128.62, 136.50, 141.80, 142.29; MS m/z = 316, calc. 316.
2-(4-Methyl-1H-indol-3-yl)-2-phenylethanol (3l)
Dark yellow oil; IR (KBr) v (cm–1): 3085, 3328, 3445; 1H NMR (CDCl3, 250 MHz) d 2.35 (s, 3H, Ar–CH3), 2.54 (s, 1H, –OH), 4.10 (m, 2H, –CH 2 –OH), 4.42 (t, 1H, Ph–C(H)–, J = 6.7 Hz), 6.79 (s, 1H, Ar–H), 6.83–7.82 (m, 8H, Ar–H), 8.45 (s, 1H, –N(H)–); 13C NMR (CDCl3, 62.9 MHz) d 22.52, 45.60, 66.41, 108.54, 111.45, 115.43, 119.44, 120.61, 122.91, 126.30, 127.02, 127.23, 127.81, 128.65, 131.43, 136.40, 142.03; MS m/z (%): 69 (64.6), 91 (16.3), 107 (11.6), 178 (25.9), 206 (100.0), 251 (M+, 23.6), 252 (M++1, 13.3).
Conclusion
In conclusion, nanomagnesium oxide as a green material is capable to catalyze epoxide ring opening by indoles. Without using excess amount of reactants, Friedel-Crafts alkylation reaction of indoles with epoxides takes place under solvent-free condition with high regioselectivity.
Acknowledgements
We sincerely acknowledge Shiraz University for supporting this work.
References
- Li , J.T. ; Dai , H.G. ; Lin , Z.P. Prog. Chem . 2007 , 19 , 751 – 761 .
- Tomioka , Y. ; Ohkubo , K. ; Maruoka , H.J. Heterocycl. Chem . 2007 , 44 , 419 – 424 .
- Unaleroglu , C. ; Aytac , S. ; Temelli , B. Heterocycles 2007 , 71 , 2427 – 2440 .
- Huffman , J.W. ; Padgett , L.W. ; Isherwood , M.L. ; Wiley , J.L. ; Martin , B.R. Bioorg. Med. Chem. Lett . 2006 , 16 , 5432 – 5435 .
- Bandini , M. ; Melloni , A. ; Tommasi , S. ; Umani-Ronchi , A. Synlett 2005 , 8 , 1199 – 1222 .
- Kang , Q. ; Zhao , Z.-A. ; You , S.-L. J. Am. Chem. Soc . 2007 , 129 , 1484 – 1485 .
- Saracoglu , N. Top. Heterocycl. Chem . 2007 , 11 , 1 – 61
- Bandini , M. ; Melloni , A. ; Umani-Ronchi , A. Org. Lett . 2004 , 6 , 3199 – 3202 .
- Jorapur , Y.R. ; Lee , C.-H. ; Chi , D.Y. Org. Lett . 2005 , 7 , 1231 – 1236 .
- Yadav , J.S. ; Reddy , B.V.S. ; Satheesh , G. Tetrahedron Lett . 2003 , 44 , 8331 – 8334 .
- Jana , U. ; Maiti , S. ; Biswas , S. Tetrahedron Lett . 2007 , 48 , 7160 – 7163 .
- Palomo , C. ; Oiarbide , M. ; Kardak , B.G. ; Garcia , J.M. ; Linden , A. J. Am. Chem. Soc . 2005 , 127 , 4154 – 4155 .
- Lin , C. ; Hsu , J. ; Sastry , M.N.V. ; Fang , H. ; Tu , Z. ; Liu , J.-T. ; Ching-Fa , Y. Tetrahedron 2005 , 61 , 11751 – 11757
- Azizi , N. ; Arynasab , F. ; Saidi , M.R. Org. Biomol. Chem . 2006 , 4 , 4275 – 4277 .
- Bandini , M. ; Cozzi , P.G. ; Melchiorre , P. ; Umani-Ronchi , A. J. Org. Chem . 2002 , 67 , 5386 – 5389 .
- Zou , X. ; Wang , X. ; Cheng , C. ; Kong , L. ; Mao , H. Tetrahedron Lett . 2006 , 47 , 3767 – 3771 .
- Lin , S. ; You , T. Tetrahedron 2009 , 65 , 1010 – 1016 .
- Tabatabaeian , K. ; Mamaghani , M. ; Mahmoodi , N.O. ; Khorshidi , A. Tetrahedron Lett . 2008 , 49 , 1450 – 1454 .
- Das , B. ; Thirupathi , P. ; Kumar , R.A. ; Reddy , K.R. Catal. Commun . 2008 , 9 , 635 – 638 .
- Liu , Y.H. ; Liu , Q.S. ; Zhang , Z.H. Tetrahedron Lett . 2009 , 50 , 916 – 921 .
- Azizi , N. ; Mehrazma , S. ; Saidi , M.R. Can. J. Chem . 2006 , 84 5 , 800 – 803 .
- Heydari , A. ; Mehrdad , M. ; Maleki , A. ; Ahmadi , N. Synthesis 2004 , 10 , 1557 – 1558 .
- Yadav , J.S. ; Reddy , B.V.S. ; Parimala , G. J. Chem. Res. Synop . 2003 , 2 , 78 – 81 .
- Westermaier , M. ; Mayr , H. Chem. Eur. J . 2008 , 14 , 1638 – 1647 .
- Kotsuki , H. ; Teraguchi , M. ; Shimomoto , N. ; Ochi , M. Tetrahedron Lett . 1996 , 37 , 3727 – 3730 .
- Yadav , J.S. ; Reddy , B.V.S. ; Araham , S. ; Sabitha , G. Synlett 2002 , 9 , 1550 – 1552 .
- Bandini , M. ; Cozzi , P.G. ; Melchiorre , P. ; Ronchi , A.U. J. Org. Chem . 2002 , 67 , 5386 – 5389 .
- Bandgar , B.P. ; Patil , A.V. Tetrahedron Lett . 2007 , 48 , 173 – 176 .
- Hudlicky , T. ; Rinner , U. ; Finn , K.J. ; Ghiviriga , I. J. Org. Chem . 2005 , 70 , 3490 – 3499 .
- Kotsuki , H. ; Hayashida , K. ; Shimanouchi , T. ; Nishizawa , H. J. Org. Chem . 1996 , 61 , 984 – 990 .
- Kotsuki , H. ; Nishiuchi , M. ; Kobayashi , S. ; Nishizawa , H. J. Org. Chem . 1990 , 55 , 2969 – 2972 .
- Kantam , M.L. ; Chakravarti , R. ; Sreedhar , B. ; Bhargava , S. Synlett 2008 , 10 , 1449 – 1454 .
- Michalkova , A. ; Ilchenko , M. ; Gorb , L. ; Leszczynski , J. J. Phys. Chem. B 2004 , 108 , 5294 – 5303 .
- Kantam , M.L. ; Laha , S. ; Yadav , J. ; Sreedhar , B. Tetrahedron Lett . 2006 , 47 , 6213 – 6217 .
- Anders , M.W. ; Jakobson , I. Scand. J. Work Environ. Health 1985 , 11 1 , 23 – 32 .
- Bolt , H.M. ; Borlak , J.T. In Toxicology ; Marquardt , H. Academic Press 1999 , Medical, Chapter 26 645 – 654 .
- Kumar , D. ; Reddy , V.B. ; Mishra , B.G. ; Rana , R.K. ; Nadagouda , M.N. ; Varma , R.S. Tetrahedron 2007 , 63 , 3093 – 3097 .
- Yu , J.C. ; Xu , A. ; Zhang , L. ; Song , R. ; Wu , L. J. Phys. Chem. B 2004 , 108 1 , 64 – 70 .
- Li , Y.X. ; Klabunde , K.J. Langmuir 1991 , 7 7 , 1388 – 1393 .
- Lu , H.B. ; Liao , L. ; Li , H. ; Tian , Y. ; Li , J.C. ; Wang , D.F. ; Zhu , B.P. J. Phys. Chem. C 2007 , 111 28 , 10273 – 10277 .
- Richards , R. ; Li , W. ; Decker , S. ; Davidson , C. ; Koper , O. ; Zaikovski , V. ; Volodin , A. ; Rieker , T. ; Klabunde , K.J. J. Am. Chem. Soc . 2000 , 122 20 , 4921 – 4928
- Zhou , Q. ; Yang , J.W. ; Wang , Y.Z. ; Wu , Y.H. ; Wang , D.Z. Mater. Lett . 2008 , 62 , 1887 – 1897 .
- J. Hu , K. Zhu , L. Chen , C. Kbel , R. Richards , J. Phys. Chem. C 2007 , 111 32 , 12038 – 12044 .
- Stark , J.V. ; Klabunde , K.J. Chem. Mater . 1996 , 8 8 , 1913 – 1918 .
- Hosseini-Sarvari , M. ; Sharghi , H. J. Iran. Chem. Soc . 2008 , 5 , 384 – 393 .
- Hosseini-Sarvari , M. ; Sharghi , H. J. Org. Chem . 2006 , 71 , 6652 – 6654
- Hosseini-Sarvari , M. ; Etemad , S. Tetrahedron 2008 , 64 , 5519 – 5523 .
- Hosseini-Sarvari , M. ; Sodagar , E. ; Doroodmand , M.M. J. Org. Chem . 2011 , 76 , 2853 – 2859
- Hosseini-Sarvari , M. Catalysis Lett . 2011 , 114 , 347 – 355 .
- Hosseini-Sarvari , M. Chin. Chem. Lett . 2011 , 22 , 547 – 554 .
- Hosseini-Sarvari , M. J. Iran. Chem. Soc . 2011 , 8 , s119 – s128 .
- Hosseini-Sarvari , M. ; Safary , E. J. Sulfur Chem . 2011 , 37 , 463 – 473 .
- Niu , H. ; Yang , Q. ; Tang , K. ; Xie , Y. Scripta Materialia 2006 , 54 , 1791 – 1725 .
- Culity , B.D. Elements of X-Ray Diffraction ; New York : Addison-Wesley , 1978 1 .
- Snyder , L.R. J. Chromatography A , 1967 , 28 , 300 – 316
- Drexler , M.T. ; Amiridis , M.D. J. Catal . 2003 , 214 , 136 – 145 .
- Yoshihiko , I. Bull. Chem. Soc. Jpn . 1984 , 57 , 73 – 84 .