Abstract
A short library of symmetrical bis-imines has been constructed efficiently from the reaction between dialdehydes and mono amines or diamines and mono aldehydes under microwave irradiation catalyzed by p-toluenesulphonic acid. The methodology is associated with shorter reaction time, good yields and simple workup.
Introduction
Imines are valuable functional groups or chemical compounds containing a carbon nitrogen double (C=N) bond. Condensation reaction of aldehydes and primary amines resulting imines, commonly called Schiff bases (first discovered by Schiff in 1864) or azomethines are well known in organic synthesis. In inorganic chemistry, these versatile Schiff bases are used as chelate for metal ion complexation Citation1–6. Some of these metal complexes are used as catalysts in various organic reactions Citation7 Citation8. Biologically, imines show anti-convulsant, antidepressant, analgesic, anti-inflammatory, antiplatelet, antimalarial, antimicrobial, antimycobacterial, and antiviral activity Citation9–11. Similar to imines, bis-imines (bis-Schiff bases) also finds use as analytical, medicinal, polymer, and liquid crystalline materials Citation12–14. Most of the biologically active nitrogen containing heterocyclic compounds and biologically active inorganic metal complexes are synthesized using bis-imines as starting materials and as synthetic intermediates Citation15–19.
The C=N bond in imines often suffers exchange reaction Citation20 as well as hydrolysis. Such reversible C=N bond formation Citation21 Citation22 is very useful to synthesize the most thermodynamically stable macrocyclic Citation23–27 and interlocked compounds Citation28 Citation29. Also, literatures are available on the heterocyclic substituted bis-imines and some of these fused bis-imines showed bio-activity Citation30, for example, bis-imines of isatin and their derivatives are known to possess a wide spectrum of pharmacological properties including antibacterial, antifungal, anti-HIV, and antiviral activity Citation31–39.
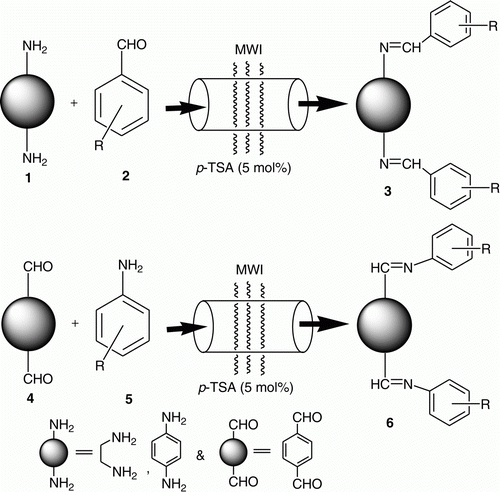
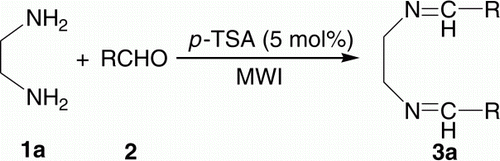
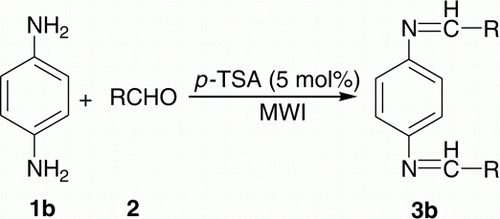
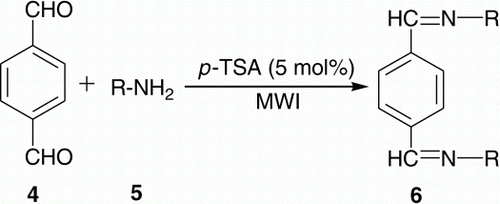
Different types of bis-imines were required in one of our ongoing programs. The conventional synthesis of such compounds involves longer reaction time using volatile organic solvents followed by extensive separation and/or purification Citation40–49. Moreover, the methods are not general. As a part of our green chemistry program and practice Citation50–52, in embracing the principles of green chemistry Citation53–55, herein we want to divulge a simple and general approach for the synthesis of bis-imines 3 and 6, by using 5 mol% p-toluenesulphonic acid (TSA) as a cheap catalyst under microwave irradiation (MWI) without using any solvent (), thereby paving the way for an environmentally benign condition. As shown in , synthesis of bis-imines involves the reaction between either diamine and mono aldehydes or dialdehyde and mono amines.
Use of Microwave energy for the enhancement of organic reactions, that is Microwave Organic Reactions Enhancement provides greener reaction condition coupled with clean product, increase yield and better time economy Citation56–63. The present investigation is aimed at looking alternative conditions for the synthesis of bis-imines with the following objectives: Citation1 search for milder reaction condition, Citation2 to reduce the reaction time, Citation3 to increase the yield, Citation4 to promote economical and environmental friendly experimental (green procedures) by performing the reaction under microwave and solvent-less/free condition, Citation5 to use cost-effective, mild, and economical catalysts.
Results and discussion
An inspection of the literatures revealed that synthesis of bis-imine was accomplished by stirring a mixture of aldehyde derivatives and aryl/alkyl diamine (or amine derivatives and aryl/alkyl dialdehyde) in the presence of anhydrous MgSO4 in dry dichloromethane at room temperature for about 15 hours Citation40 or refluxing the reactants in ethanolic solution and toxic solvent like benzene Citation41 along with other reported reaction conditions Citation42–44. However, the reported procedures have some drawbacks such as low yield, longer reaction time, lack of generality, harsh reaction condition, use of costly and environmentally harmful catalysts and so on. In recent years, due to consciousness and legislature, environmentally benign synthetic methods have received a considerable attention. Varma et al. Citation64 reported clay catalyzed synthesis of monoimines and enamines under solvent-free conditions using MWI. The condensation reaction of aldehyde and amines in water suspension medium was reported by Tanaka et al. Citation65. Ancker et al. Citation66 described synthesis of bis-imines by Citation1 a solvent-free method, grinding and, Citation2 using PEG (polyethylene glycol) as a benign reaction medium. A convenient condensation of the fluorinated β-hydroxy-β-bis(trifluoromethyl)-ketones with primary amines or diamines was achieved in the presence of Lewis acid (InBr3, La(OTf)3), or Brönsted (PTSA) acid catalysts Citation67. In this regard, hence, looking at the wide spectrum application of bis-imines in the field of medicinal, organic, inorganic, and material chemistry, there is still a demand for developments of alternative methodology for the synthesis of bis-imines, which is quite general associated with simple reaction condition, short reaction time and high yields. As part of our green chemistry program Citation50–52, in this approach, we wanted to search for the conditions where the substrates would be used in stoichiometric ratios and the catalyst used would be in minimum amount. We have focused on p-TSA, which is cheap, easily available and can be easily removed from the reaction mixture being water-soluble Citation51. We studied the effects of p-TSA, under thermal as well as MWI in the absence of solvent and in the presence of solvent to optimize the reaction between ethylene diamine (EDA) and p-chlorobenzaldehyde (), p-phenylenediamine (p-PDA) and p-chlorobenzaldehyde (), terephthaldehyde and p-chloroaniline () were chosen as the model reactions. From the environmental considerations, we have checked the reactions in ethanol, Methanol, and ethyl acetate. Halogenated solvents and benzene were not considered. However, solvent-free reactions were most encouraging. Under MWI, within few minutes we got the products with excellent to very good yields as compared to several hours under classical heating condition, so we continued our generalization under MWI and those results have been discussed here.
Different amounts of catalyst loading were tested. The results are shown in . In the absence of catalyst, the reaction proceeded to give poor yields. Using catalyst the yields were improved. Consequently, 5 mol% of p-TSA was found to be the optimum one. Similarly, different MW power and reaction temperature in each of the schemes were also tested. The results are shown in .
Table 1. Effect of catalyst p-toluenesulphonic acid loading with MW power, time, and temperature on the yields of bis-imines 3a, 3b, and 6.
In order to extend the scope and generality of the reactions and to have a short library of bis-imines we varied both the amine and aldehyde part. Synthesis of bis-imines 3a from EDA 1a and aldehyde derivatives 2 () has been elaborated in (entries 1–13). Good to excellent yields (61–99%) of the products were obtained. As compared to aromatic ones (entries 1–10, ), aliphatic aldehydes provided somewhat lower yields (61–63%) of the products (entries 11–13, ). (entries 1–13) summarizes the results of the reaction between p-PDA 1b and aldehyde derivatives 2 () providing 69–99% yield of the products 3b. Similar to , here also aliphatic aldehydes (entries 11–13, ) provided somewhat lower yields (69–72%) as compared to aromatic ones.
Table 2. Synthesis of bis-imines 3a from EDA 1a and aldehyde derivatives 2.
Table 3. Synthesis of bis-imines 3b from p-PDA 1b and aldehyde derivatives 2.
We also checked the reaction of terephthaldehyde 4 with amine derivatives 5 to obtain bis-imines 6 () which gave 71–92% yields. Summary of the synthesis of 6 has been described in (entries 1–9).
Table 4. Synthesis of bis-imines 6 from terephthaldehyde 4 and amine derivatives 5.
As can be seen from and , and , the reaction appears to be quite general and diverse types of bis-imines can be obtained by varying the amine part or the aldehydic part. The reported method Citation66 describes the bis-imine synthesis starting from two aliphatic aldehydes (EDA and cyclohexyldiamine) only. Several pharmacologically important moieties can be introduced into the imines, for example, −Cl, −NO2, −Me, −OMe, −OH, double bond and so on, showing their tolerance to the reaction condition. Aliphatic and aromatic aldehydes and amines responded equally to the reaction. As reported by Fujioka et al. Citation82 and Gogoi et al. Citation83 where reaction between EDA and aldehyde derivatives afforded 2-dihydroimidazole derivatives, however, in our case we did not observe such product. Notably, present method of synthesis of bis-imines reduces the reaction time from several hours to few minutes. Using p-TSA as catalyst, we received excellent to good yields of the products. Being water soluble, the catalyst can be easily removed from the reaction mixture making the purification easier-an important point in the context of green chemistry. Finally, recrystallization from ethanol furnished the pure products. Overheating and increase in the reaction time did not increase the yield; rather we got some insoluble waste materials.
The products were supported by the absence of the carbonyl and primary amine bands of the reactants in their IR spectra, and the presence of an imine ν (C=N) band within 1613–1636 cm−1 region. In the 1H NMR spectra, the azomethine (CH=N) protons appear within δ=8.00–9.00 ppm. In the 13C NMR spectra, the imine carbon appears within 150.00–170.00 ppm. Progress of the reaction can be easily monitored by IR and 1H NMR spectroscopy in addition to Thin Layer Chromatography (TLC). In IR spectra, appearance of C=N peak and disappearance of >C=O peak and in 1H NMR spectra appearance of azomethine (CH=N)proton are the focusing point.
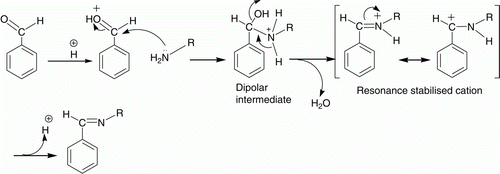
A plausible mechanism for the reaction is shown in . Mechanistically, the acidic proton of catalyst protonates the oxygen atom of carbonyl group. Protonation activates carbonyl group toward nucleophilic addition. Nucleophilic nitrogen of amine then attacks the electrophilic carbon center of aldehyde group to form a dipolar intermediate, which releases water to form a resonance stabilized cation. Finally, elimination of a proton from this cation afforded the product, imine.
The procedure described above has several advantages over existing methods for the synthesis of bis-imines. The use of any drying agents or apparatus to remove the water formed during the reaction is not necessary. Time economy observed is amazing and also p-TSA as catalyst is very cheap and effective, which can be easily removed from the reaction mixture. Moreover, use of “MWI” considerably improves the yield of the products as compared to the “classical heating.”
Different steps (reaction, separation, and purification) in a synthesis contribute to the environmental footprint of the process Citation84. Since, our method reduces the amount of solvent used, hence the methodology is environmentally benign. Owing to the important precursors for the synthesis of several biologically versatile heterocyclic rings, our finding may be helpful.
In conclusion, a short library of symmetrical bis-imines has been constructed from the reaction between amines and aldehydes under MWI.
Experimental
Melting points were determined in open capillary tubes on a Büchi 504 apparatus and are uncorrected. IR spectra were recorded in KBr pallets on a Nicolet (Impact 410) FT-IR spectrophotometer. 1H NMR and 13C NMR spectra were recorded on a JNM ECS 400 MHz NMR spectrophotometer (JEOL) using Tetramethylsilane as the internal standard. Coupling constants are expressed in Hertz. Elemental analyses were carried out in a Perkin Elmer CHN analyzer (2400 series II). Mass spectra were recorded on a Waters Q-TOF Premier & Aquity UPLC spectrometer. Microwave synthesis system used was model CAT-2R from CatalystTM systems equipped with an external surface sensor. The reactions were monitored by thin layer chromatography using aluminium sheets with silica gel 60F254 (Merck). All the chemicals used as received.
General experimental procedure for the synthesis of bis-imines 3a
Typically, a mixture of EDA (0.134 ml, 2 mmol), benzaldehyde (0.406 ml, 4 mmol), and pure p-TSA (19 mg, 5 mol%) were taken in a 25 ml Erlenmeyer flask and irradiated in a microwave synthesis system (600W) for 10 min. The reaction was monitored by TLC. The crude product was dissolved in minimum amount of ethyl acetate and upon addition of hexane, bis-imine precipitated out. The precipitate was filtered and then dried in vacuum to afford the bis-imine (0.178 g, 75% yield) melting point 53.6–54.3°C. The pure products were characterized by IR, 1H-NMR, 13C-NMR spectroscopy, mass spectrometry, and comparison of their melting points with the reported one.
Same procedure was used for the synthesis of bis-imines 3b and 6.
Supplementary material
Physical and spectral data of the synthesized compounds are given as Supplementary material.
Acknowledgements
AJT thanks Department of Science and Technology (DST), Government of India, New Delhi for financial support to the project (Grant no. SR/FTP/CS-58/2005) and SD thanks University Grant Commission (UGC), Government of India, New Delhi for the Rajiv Gandhi National Fellowship. LS thanks Council of Scientific and Industrial research (CSIR), Government of India, New Delhi for financial support as research fellowship.
Authors are thankful to University Grant Commission, New Delhi-Special Assistance Program (UGC-SAP) [no. F.3-30/2009(SAP-II) dated 31.03.2009] and Department of Science and Technology- Fund for Improvement of S&T Infrastructure in Higher Educational Institutions (DST-FIST) program [no. SR/FST/CS-II-001/2009 dated 17.11.2009] for providing infrastructure facilities required for this research work.
References
- Layer , R.W. Chem. Rev . 1963 , 63 , 489 .
- Alexander , V. Chem. Rev . 1995 , 95 , 273 .
- Borisova , N.E. ; Reshetova , M.D. ; Ustynyuk , Y.A. Chem. Rev . 2007 , 107 , 46 .
- Roland S. ; Mangeney , P. Eur. J. Org. Chem . 2000 , 4 , 611 .
- Cerchiaro , G. ; Micke , G.A. ; Tavares , M.F.M. ; Ferriera , A.M.D.C. J. Mol. Catal. A: Chem . 2004 , 221 , 29 .
- Juana , Z. ; Lua , C. ; Haihonga , W. ; Jianguo , Y. Chinese J. Chem . 2009 , 27 , 1868 .
- Jacobsen , E.N. Catalytic Asymmetric Synthesis ; Ed.: Ojima , I. New York : VCH , 1993 .
- Katsuki , T. Coord. Chem. Rev . 1995 , 140 , 189 .
- Rollas , S. ; Kucukguzel , S.G. Mol . 2007 , 12 , 1910 .
- Piato , A.L. ; Detanico , B.C. ; Jesus , J.F. ; Lhullier , F.L. ; Nunes , D.S. ; Elisabetsky , E. J. Ethnopharmacol . 2008 , 118 , 300 .
- Lidija , B.R. Acta. Pharm . 2004 , 54 , 157 .
- Higuchi , M. ; Yamamoto , K. Org. Lett . 1999 , 1 , 1881 .
- Adams , J.P. J. Chem. Soc., Perkin Trans . 2000 , 1 , 125 .
- Naeimi , H. ; Moradian , M. J. Coord. Chem . 2010 , 63 , 156 .
- Silva , J.F.M.D. ; Garden , S.J. ; Pinto A.C. J. Braz. Chem. Soc . 2001 , 12 , 273 .
- Shi , M. ; Cui , S.C. New J. Chem . 2004 , 28 , 1286 .
- Jia , X. ; Lin , H. ; Huo , C. ; Zhang , W. ; Lu , J. ; Yang , L. ; Zhao , G. ; Liu , Z. Synlett . 2003 , 11 , 1707 .
- Behforouz , M. ; Ahmadian , M. Tetrahedron 2000 , 56 , 5259 .
- Zulfiqar , F. ; Kitazume , T. Green Chem . 2000 , 2 , 137
- Cordes , E.H. ; Jencks , W.P. J. Am. Chem. Soc . 1962 , 84 , 832 .
- For reviews, see: Layer, R.W . Chem. Rev . 1963 , 63 , 489 .
- Rowan , S.J. ; Cantrill , S.J. ; Cousins , G.R.L. ; Sanders , J.K.M. ; Stoddart , J.F. Angew. Chem., Int. Ed . 2002 , 41 , 898 .
- Schmidt , S. ; Bauer , W. ; Heinemann , F.W. ; Lanig , H. ; Grohmann , A. Angew. Chem., Int. Ed . 2000 , 39 , 913 .
- Gawronski , J. ; Kolbon , H. ; Kwit , M. ; Katrusiak , A. J. Org. Chem . 2000 , 65 , 5768 .
- Akine , S. ; Taniguchi , T. ; Nabeshima , T. Tetrahedron Lett . 2001 , 42 , 8861 .
- Zhao , D. ; Moore , J.S. J. Org. Chem . 2002 , 67 , 3548 .
- Akine , S. ; Hashimoto , D. ; Saiki , T. ; Nabeshima , T. Tetrahedron Lett . 2004 , 45 , 4225 .
- Cantrill , S.J. ; Rowan , S.J. ; Stoddart , J.F. Org. Lett . 1999 , 1 , 1363 .
- Rowan , S.J. ; Stoddart , J.F. Org. Lett . 1999 , 1 , 1913 .
- Venugopala , K.N. ; Rao , G.K. ; Pai , P.N.S. J. Pharmacol. Toxicol . 2007 , 2 , 248 .
- Jarrahpour , A. ; Khalili , D. ; Clercq , E.D. ; Salmi , C. ; Brunel , J.M. Molecules 2007 , 12 , 1720 .
- Pandeya , S.N. ; Sriram , D. Acta Pharm. Turc . 1998 , 40 , 33 .
- Sarangapani , M. ; Reddy , V.M. Ind. J. Heterocycl. Chem . 1994 , 3 , 257 .
- Sridhar , S.K. ; Pandeya , S.N. ; Stables , J.P. ; Ramesh , A. Eur. J. Pharm. Sci . 2002 , 16 , 129 .
- Varma , M. ; Pandeya , S.N. ; Singh , K.N. ; Stables , J.P. Acta Pharmaceut . 2004 , 54 , 49 .
- Pandeya , S.N. ; Sriram , D. ; Nath , G. ; Clercq , E.D. Ind. J. Pharm. Sci . 1983 , 52 , 766 .
- Elmali , F.T. ; Peksel , A. ; Demirhan , N. J. Coord. Chem . 2009 , 62 , 2548 .
- Tumerdem , G.T. ; Turgut , Y. Tetrahedron: Asymmetry 2005 , 16 , 865 .
- Lewkowski , J. ; Rzezniczak , M. ; Skowronski , R. Heteroatom Chem . 2000 , 11 , 144 .
- Karupaiyan , K. ; Puranik , V.G. ; Deshmukh , A.R.A.S. ; Bhawal , B.M. Tetrahedron 2000 , 56 , 8555 .
- Sharma , V. ; Khan , M.S.Y. Eur. J. Med. Chem . 2001 , 36 , 65 .
- Shakil , N.A. ; Dhawan , A. ; Sharma , N.K. ; Kumar , V. ; Kumar , S.M. ; Raj , H.G. ; Olsen , C.E. ; Cholli , A.K. ; Samuelson , L.A. ; Kumar , J. ; Watterson , A.C. ; Parmer , V.S. ; Prasad , A.K. Ind. J. Chem . 2003 , 42 ( B ), 1958 .
- Tumerdem , G.T. ; Turgut , Y. Tetrahedron: Asymmetry 2005 , 16 , 865 .
- Lewkowski , J. ; Rzezniczak , M. ; Skowronski , R. Heteroatom Chem . 2000 , 11 , 144 .
- Ahamad , T. ; Nishat , N. ; Parveen , S. J. Coord. Chem . 2008 , 61 , 1963 .
- Salerno , A. ; Figueroa , A.M. ; Perillo , I.A. Synth. Commun . 2003 , 33 , 3193 .
- Billman , J.H. ; Ho , J.Y.C. ; Caswell , L.R. J. Am. Chem. Soc . 1952 , 17 , 1375 .
- van Alphen , J. Rec. Trav. Chim . 1935 , 54 , 885 .
- Billman , J.H. ; Chem Ho , J.J. ; Caswell , L.R. J. Org. Chem . 1957 , 22 , 538 .
- Das , S. ; Thakur , A.J. Eur. J. Org. Chem . 2011 , 2 , 2301 .
- Das , S. ; Thakur , A.J. Green Chem. Lett. Rev . 2011 , 4 ( 2 ), 131 .
- Saikia , L. ; Das , S. ; Thakur , A.J. Synth. Commun . 2011 , 41 , 1071 .
- Anastas , P.T. ; Warner , J.C. In Green Chemistry: Theory and Practice ; New York : Oxford Publications , 1998 .
- Anastas , P.T. ; Williamson , T. Green Chemistry, Frontiers in Benign Chemicals Synthesis and Processes ; New York : Oxford Science publications , 1998 .
- Tanaka , K. Solvent free Organic Synthesis ; Weinheim : Wiley-VCH , 2003 .
- Prajapati , D. ; Gohain , M. ; Thakur , A. J. Bioorg. Med. Chem. Lett . 2006 , 16 , 3537 .
- Thakur , A.J. ; Prajapati , D. Tetrahedron Lett . 2005 , 34 , 1433 .
- For reviews see : Caddick , S. Tetrahedron 1995 , 51 , 10403 .
- Loupy , A. ; Petit , A. ; Hamelin , J. ; Texier-Boullet , J. ; Jacquault , F. ; Methe , D. Synthesis 1998 , 9 , 1213 .
- Galema , S.A. Chem. Soc. Rev . 1997 , 26 , 233 .
- Satyanarayana , V.S.V. ; Sreevani , P. ; Sivakumar , A. ; Vijayakumar , V. Arkivoc 2008 , xvii , 221 .
- Ali , P. ; Ramakanth , P. ; Meshram , J. J. Coord. Chem . 2010 , 63 , 323 .
- Polshettiwar , V. ; Varma , R.S. Acc. Chem. Res . 2008 , 41 , 629 .
- Varma , R.S. ; Dahiya , R. ; Kumar , S. Tetrahedron Lett . 1997 , 38 , 2039 .
- Tanaka , K. ; Shiraishi , R. Green Chem . 2000 , 2 , 272 .
- van den Ancker , T.R. ; Caveb , G.W.V. ; Rastonc , C.L. Green Chem . 2006 , 8 , 50 .
- Marquet , N. ; Grunova , E. ; Kirillov , E. ; Bouyahyi , M. ; Thomas , C.M. ; Carpentier , J-F. Tetrahedron 2008 , 64 ( 1 ), 75 .
- Frost , A.E. J. Org. Chem . 1959 , 24 , 1905 .
- Amirnasr , M. J. Coord. Chem . 2003 , 56 ( 3 ), 231 .
- Bahron , H. Acta Cryst . 2007 , E63 ( 2 ), o558 .
- Mishra , L. Spectrochimica Acta , 2008 , 70A ( 1 ), 79 .
- Bomfim , J.A.S. Acta Cryst . 2005 , C61 ( 1 ), o53 .
- Syuleiman , T. Godishnik na Visshiya Khimiko-Tekhnologichen Institut: Burgas ; 1981 , 15 , Pt. 2 , 91 .
- He , J.-Y. Ultrason. Sonochem . 2010 , 18 ( 1 ), 466 .
- Gioia , D. ; Luisa , M. J. Chromatography, A 2005 , 1066 ( 1–2 ), 143 .
- Wang , X.-G. ; Shandong , D.X. Lixueban 2009 , 44 ( 7 ), 18 .
- Mohan , J. Ind. J. Het. Chem . 2004 , 13 ( 4 ), 327 .
- Kiviranta , P.H. J. Med. Chem . 2006 , 49 ( 26 ), 7907 .
- Senier , A. J. Chem. Soc., Trans . 1909 , 95 , 1943 .
- Gyul'akhmedov , L.M. Uch. Zap. Azerb. Un-t. Ser. Khim. N . 1978 , 4 , 46 .
- Collas , A. Acta Cryst . 2011 , C67 ( 5 ), o171 .
- Fujioka , H. ; Murai , K. ; Ohba , Y. ; Hiramatsu , A. ; Kita ; Y. Tetrahedron Lett . 2005 , 46 , 2197 .
- Gogoi , P. ; Konwar , D. Tetrahedron Lett . 2006 , 47 , 79 .
- Pollet , P. ; Hart , R.J. ; Eckert , C.A. ; Liotta , C.L. Acc. Chem. Res . 2010 , 43 ( 9 ), 1237 .
Supplementary information
Physical and Spectral data for the bis-imines
3a(1). N1,N2 -dibenzylideneethane-1,2-diamine (entry 1, table 1)
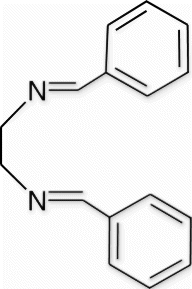
Yield 75%, m.p. 53.6–54.3 °C, 1H NMR (400 MHz, CDCl3) δH: 3.94 (s, 4H, 2CH2), 7.34–7.36 (m, 6H, arom), 7.65–7.67 (m, 4H, arom), 8.25 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 61.8, 128.4, 128.7, 130.8, 136.3, 162.9; IR (KBr) νmax: 1637.09 (−CH=N−) cm−1; MS, m/z 237.13 (M+); Anal. Calcd (%) for C16H16N2: C, 81.32; H, 6.82; N, 11.85. Found C, 81.33; H, 6.85; N, 11.86.
3a(2). N1,N2 -bis(3-phenylallylidene)ethane-1,2-diamine (entry 2, table 1)
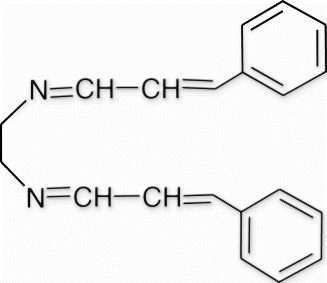 Yield 99%, m.p. 101.7–103.6 °C, 1H NMR (400 MHz, CDCl3) δH: 3.84 (s, 4H, 2CH2), 6.85–6.96 (m, 4H, 2CH=CH), 7.29–7.37 (m, 6H, arom), 7.44–7.47 (m, 4H, arom), 8.04 (d, 2H, J=7.32, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 61.5, 127.2, 127.6, 128.5, 12.78, 135.6, 142.0, 164.31; IR (KBr) νmax: 1638.12 (−CH=N−) cm−1; MS, m/z 288.16 (M+); Anal. Calcd (%) for C20H20N2: C, 83.30; H, 6.99; N, 9.71. Found C, 83.34; H, 7.03; N, 9.69.
Yield 99%, m.p. 101.7–103.6 °C, 1H NMR (400 MHz, CDCl3) δH: 3.84 (s, 4H, 2CH2), 6.85–6.96 (m, 4H, 2CH=CH), 7.29–7.37 (m, 6H, arom), 7.44–7.47 (m, 4H, arom), 8.04 (d, 2H, J=7.32, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 61.5, 127.2, 127.6, 128.5, 12.78, 135.6, 142.0, 164.31; IR (KBr) νmax: 1638.12 (−CH=N−) cm−1; MS, m/z 288.16 (M+); Anal. Calcd (%) for C20H20N2: C, 83.30; H, 6.99; N, 9.71. Found C, 83.34; H, 7.03; N, 9.69.
3a(3). N1,N2 -bis(4-methoxybenzylidene)ethane-1,2-diamine (entry 3, table 1).
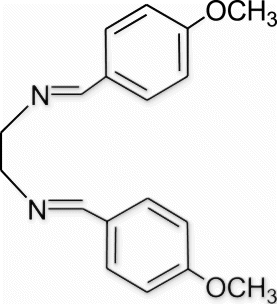 Yield 98%, m.p. 107.5–109.6 °C, 1H NMR (400 MHz, CDCl3) δH: 3.76 (s, 6H, 2OCH3), 3.91 (s, 4H, 2CH2), 6.89 (d, 4H, J=8, arom), 7.64 (d, 4H, J=8.8, arom), 8.20 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 55.4, 61.8, 128.3, 128.7, 130.8, 136.3, 162.9; IR (KBr) νmax: 1641.12 (−CH=N−) cm−1; MS, m/z 296.15 (M+); Anal. Calcd (%) for C18H20N2O2: C, 72.95; H, 6.80; N, 9.45. Found C, 72.99; H, 6.84; N, 9.46.
Yield 98%, m.p. 107.5–109.6 °C, 1H NMR (400 MHz, CDCl3) δH: 3.76 (s, 6H, 2OCH3), 3.91 (s, 4H, 2CH2), 6.89 (d, 4H, J=8, arom), 7.64 (d, 4H, J=8.8, arom), 8.20 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 55.4, 61.8, 128.3, 128.7, 130.8, 136.3, 162.9; IR (KBr) νmax: 1641.12 (−CH=N−) cm−1; MS, m/z 296.15 (M+); Anal. Calcd (%) for C18H20N2O2: C, 72.95; H, 6.80; N, 9.45. Found C, 72.99; H, 6.84; N, 9.46.
3a(4). N1,N2 -bis(4-chlorobenzylidene)ethane-1,2-diamine (entry 4, table 1)
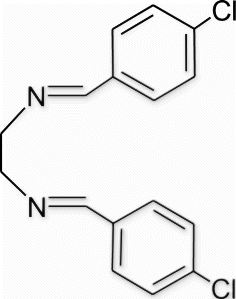
Yield 99%, m.p. 149.1–150.3 °C, 1H NMR (400 MHz, CDCl3) δH: 3.97 (s, 4H, 2CH2), 7.36 (d, 4H, J=8.24, arom), 7.64 (d, 4H, J=8.24, arom), 8.24 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 61.5, 128.5, 128.9, 134.6, 136.6, 161.4; IR (KBr) νmax: 1637.09 (−CH=N−) cm−1; MS, m/z 304.05 (M+); Anal. Calcd (%) for C16H14 Cl2N2: C, 62.97; H, 4.62; N, 9.18. Found C, 62.99; H, 4.66; N, 9.16.
3a(5). N1,N2 -bis(2-chlorobenzylidene)ethane-1,2-diamine (entry 5, table 1)
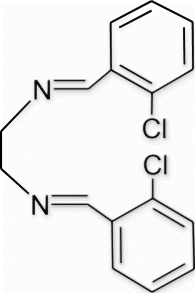
Yield 98%, m.p. 148.4–149.7 °C, 1H NMR (400 MHz, CDCl3) δH: 3.98 (s, 4H, 2CH2), 7.37–7.39 (m, 6H, arom), 7.65 (d, 2H, J=8.24, arom), 8.24 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 61.6, 128.3, 28.8, 131.3, 132.6, 133.9, 136.5, 162.0; IR (KBr) νmax: 1637.21 (−CH=N−) cm−1; MS, m/z 304.05 (M+); Anal. Calcd (%) for C16H14 Cl2N2: C, 62.97; H, 4.62; N,9.18. Found C, 63.00; H, 4.66; N, 9.18.
3a(6). N1,N2 -bis(4-hydroxybenzylidene)ethane-1,2-diamine (entry 6, table 1)
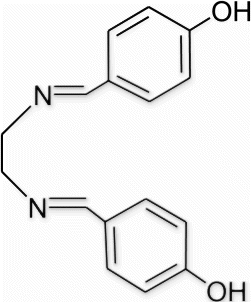
Yield 95%, m.p. 126.7–127.4 °C, 1H NMR (400 MHz, CDCl3) δH: 3.93 (s, 4H, 2CH2), 6.89 (d, 4H, J=8.24, arom), 7.31 (d, 4H, J=8.72, arom), 8.31 (s, 2H, 2N=CH), 9.37 (s, 2H, 2OH); 13C NMR (100 MHz, CDCl3) δc: 61.7, 128.1, 128.4, 131.1, 135.9, 162.7; IR (KBr) νmax: 3479.21 (−OH), 1637.18 (−CH=N−) cm−1; MS, m/z 268.12 (M+); Anal. Calcd (%) for C16H16N2O2: C, 71.62; H, 6.01; N, 10.44. Found C, 71.61; H, 6.04; N, 10.42.
3a(7). N1,N2 -bis(2-hydroxybenzylidene)ethane-1,2-diamine (entry 7, table 1)
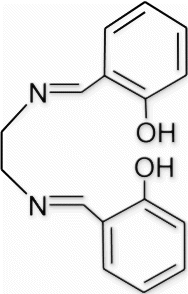
Yield 100%, m.p. 125.9–127.3 °C, 1H NMR (400 MHz, CDCl3) δH: 3.93 (s, 4H, 2CH2), 6.83–6.87 (m, 2H, arom), 6.93 (d, 2H, J=8.4, arom), 7.23–7.30 (m, 4H, arom), 8.35 (s, 2H, 2N=CH), 13.21 (s, 2H, 2OH); 13C NMR (100 MHz, CDCl3) δc: 61.6, 128.3, 128.7, 129.9, 130.8, 132.5, 136.3, 162.9; IR (KBr) νmax: 3477.16 (−OH), 1667.89 (−CH=N−) cm−1; MS, m/z 268.12 (M+); Anal. Calcd (%) for C16H16N2O2: C, 71.62; H, 6.01; N, 10.44. Found C, 71.61;H, 6.04; N, 10.41.
3a(8). N1,N2 -bis(4-methylbenzylidene)ethane-1,2-diamine (entry 8, table 1)
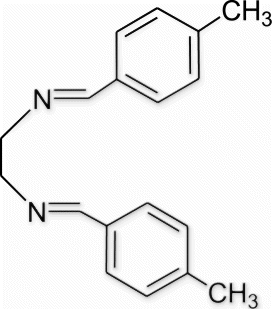
Yield 98%, m.p. 159.7–160.2 °C, 1H NMR (400 MHz, CDCl3) δH: 2.35 (s, 6H, 2CH3), 3.92 (s, 4H, 2CH2), 7.19 (d, 4H, J=6.88, arom), 7.55 (d, 4H, J=6.88, arom), 8.23 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 28.8, 61.6, 128.0, 129.2, 133.5, 140.7, 162.5; IR (KBr) νmax 1636.87 (−CH=N−) cm−1; MS, m/z 264.16 (M+); Anal. Calcd (%) for C18H20N2: C, 81.78; H, 7.63; N, 10.60. Found C, 81.79; H, 7.66; N, 10.57.
3a(9). N1,N2 -bis(4-nitrobenzylidene)ethane-1,2-diamine (entry 9, table 1)
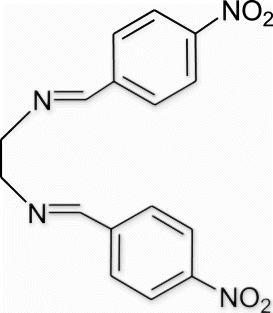
Yield 83%, m.p. 161.7–162.9 °C, 1H NMR (400 MHz, CDCl3) δH: 3.94 (s, 4H, 2CH2), 7.87 (d, 4H, J=7.32, arom), 8.21 (d, 4H, J=7.32, arom), 8.45 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 61.5, 127.7, 128.3, 132.8, 147.9, 161.3; IR (KBr) νmax: 1636.89 (−CH=N−) cm−1; MS, m/z 326.10 (M+); Anal. Calcd (%) for C16H14N4O4: C, 58.89; H, 4.32; N, 17.17. Found C, 58.91; H, 4.35; N, 17.14.
3a(10). N1,N2 -bis(3-nitrobenzylidene)ethane-1,2-diamine (entry 10, table 1)
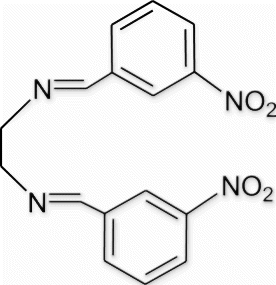
Yield 98%, m.p. 162.5–164.1 °C, 1H NMR (400 MHz, CDCl3) δH: 4.03 (s, 4H, 2CH2), 7.56–7.59 (m, 2H, arom), 8.03 (d, 2H, J=7.32, arom), 8.24–8.38 (m, 4H, arom), 8.53 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 61.44, 123.00, 125.32, 129.85, 133.84, 137.86, 148.79, 160.27; IR (KBr) νmax: 1637.06 (−CH=N−) cm−1; MS, m/z 326.10 (M+); Anal. Calcd (%) for C16H14N4O4: C, 58.89; H, 4.32; N, 17.17. Found C, 58.91; H, 4.36; N, 17.15.
3a(11). N1,N2 -diethylideneethane-1,2-diamine (entry 11, table 1)
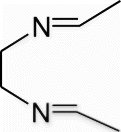
Yield 27%, m.p. 103.5–104.8 °C, 1H NMR (400 MHz, CDCl3) δH: 1.21 (d, 6H, J= 8.4, CH3), 3.93 (s, 4H, 2CH2), 8.23 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 16.1, 62.0, 158.6; IR (KBr) νmax: 1643.06 (−CH=N−) cm−1; MS, m/z 113.10 (M+); Anal. Calcd (%) for C6H12N2: C, 64.24; H, 10.78; N, 24.97. Found C, 64.28; H, 10.76; N, 24.95.
3a(12). N1,N2 -dibutylideneethane-1,2-diamine (entry 12, table 1)
Yield 31%, m.p. 98.6–101.3 °C, 1H NMR (400 MHz, CDCl3) δH: 1.17–1.21 (m, 6H, CH3), 1.67–2.24 (m, 8H, 4CH2), 3.97 (s, 4H, 2CH2), 8.24 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 16.2, 27.1, 28.7, 61.5, 159.2; IR (KBr) νmax 1643.06 (−CH=N−) cm−1; MS, m/z 169.17 (M+); Anal. Calcd (%) for C10H20N2: C, 71.37; H, 11.98; N, 16.65. Found C, 71.39; H, 11.95; N, 16.65.
3a(13). N1,N2 -dipentylideneethane-1,2-diamine (entry 13, table 1)
Yield 31%, m.p. 87.7–89.1 °C, 1H NMR (400 MHz, CDCl3) δH: 1.03–1.09 (m, 6H, CH3), 1.55–2.14 (m, 8H, 4CH2), 2.27–2.31 (m, 4H, 4CH2), 3.97 (s, 4H, 2CH2), 8.24 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 16.1, 27.2, 27.4, 28.7, 61.5, 159.5; IR (KBr) νmax: 1637.91 (−CH=N−) cm−1; MS, m/z 197.2 (M+); Anal. Calcd (%) for C12H24N2: C, 73.41; H, 12.32; N, 14.27. Found C, 73.43; H, 12.31; N, 14.24.
3b(1). N1,N4 -dibenzylidenebenzene-1,4-diamine (entry 1, table 2)
Yield 80%, m.p. 141–141.7 °C, 1H NMR (400 MHz, CDCl3) δH: 7.28 (s, 4H, arom), 7.47–7.49 (m, 6H, arom), 7.90–7.92 (m, 4H, arom), 8.50 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 121.8, 128.7, 131.3, 136.2, 149.9, 159.7; IR (KBr) νmax: 1613.80 (−CH=N−) cm−1; MS, m/z 285.13 (M+); Anal. Calcd (%) for C20H16N2: C, 84.48; H, 5.67; N, 9.85. Found C, 84.47; H,5.76; N, 9.86.
3b(2). N1,N 4 -bis(3-phenylallylidene)benzene-1,4-diamine (entry 2, table 2)
Yield 99%, m.p. 226.9–228.3 °C, 1H NMR (400 MHz, CDCl3) δH: 7.13–7.16 (m, 4H, 2CH=CH), 7.25 (s, 4H, arom), 7.36–7.42 (m, 6H, arom), 7.56–7.54 (m, 4H, arom), 8.32 (d, 2H, J=7.32, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 122.01, 127.61, 128.70, 129.03, 129.70, 135.69, 144.11, 149.82, 161.09; IR (KBr) νmax: 1615.77 (−CH=N−) cm−1; MS, m/z 336.16 (M+); Anal. Calcd (%) for C24H20N2: C, 85.68; H, 5.99; N, 8.33. Found C, 85.67; H, 6.01; N, 8.34.
3b(3). N1,N4 -bis(4-methoxybenzylidene)benzene-1,4-diamine (entry 3, table 2)
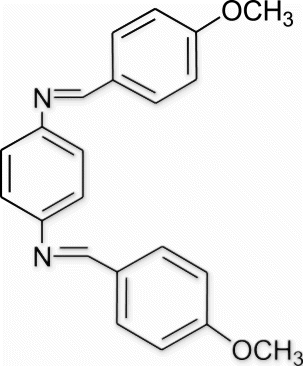
Yield 96%, m.p. 206.7–207.9 °C, 1H NMR (400 MHz, CDCl3) δH: 3.85 (s, 6H, 2OCH3), 6.96 (d, 4H, J=8.24, arom), 7.21 (d, 4H, J=8, arom), 7.83 (s, 4H, arom), 8.39 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 55.1, 114.1, 121.4, 129.0, 129.7, 149.6, 158.6, 161.9; IR (KBr) νmax: 1613.80 (−CH=N−) cm−1; MS, m/z 344.15 (M+); Anal. Calcd (%) for C22H20N2O2: C, 76.72; H, 5.85; N, 8.13. Found C, 76.75; H, 5.86; N, 8.14.
3b(4). N1,N4 -bis(4-chlorobenzylidene)benzene-1,4-diamine (entry 4, table 2)
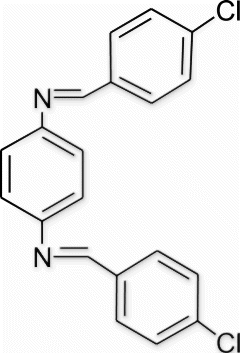
Yield 99%, m.p. 202.3–202.9 °C, 1H NMR (400 MHz, CDCl3) δH: 7.27 (s, 4H, arom), 7.44 (d, 4H, J=8.72, arom), 7.84 (d, 4H, J=8.24, arom), 8.46 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 121.9, 129.1, 130.0, 134.8, 137.4, 149.8, 158.2; IR (KBr) νmax: 1637.42 (−CH=N−) cm−1; MS, m/z 353.05 (M+); Anal. Calcd (%) for C20H14Cl2N2: C, 68.00; H, 3.99; N, 7.93. Found C, 68.01; H, 4.01; N, 7.91.
3b(5). N1,N4 -bis(2-chlorobenzylidene)benzene-1,4-diamine (entry 5, table 2)
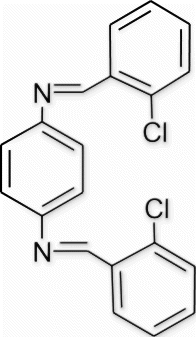
Yield 98%, m.p. 203–204.1 °C, 1H NMR (400 MHz, CDCl3) δH: 7.28 (s, 4H, arom), 7.47–7.85 (m, 8H, arom), 8.46 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 121.9, 128.8, 129.3, 129.7, 130.2, 134.8, 137.4, 149.7, 158.4; IR (KBr) νmax :1637.42 (−CH=N−) cm−1; MS, m/z 353.05 (M+); Anal. Calcd (%) for C20H14Cl2N2: C, 68.00; H, 3.99; N, 7.93. Found C, 68.03; H, 4.01; N, 7.96.
3b(6). N1,N4 -bis(4-hydroxybenzylidene)benzene-1,4-diamine (entry 6, table 2)
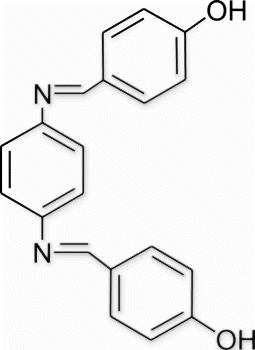
Yield 92%, m.p. 259.9–261 °C, 1H NMR (400 MHz, CDCl3) δH: 6.65–7.06 (m, 8H, arom), 7.69–7.76 (m, 4H, arom), 8.41 (s, 2H, 2N=CH), 9.78 (s, 2H, 2OH); 13C NMR (100 MHz, CDCl3) δc: 115.0, 115.6, 121.5, 121.8, 130.4, 131.9, 158.9; IR (KBr) νmax: 3533.76 (−OH), 1643.45 (−CH=N−) cm−1; MS, m/z 316 (M+); Anal. Calcd (%) for C20H16N2O2: C, 75.93; H, 5.10; N, 8.86. Found C, 75.92; H, 5.13; N, 8.89.
3b(7). N1,N4 -bis(2-hydroxybenzylidene)benzene-1,4-diamine (entry 7, table 2)
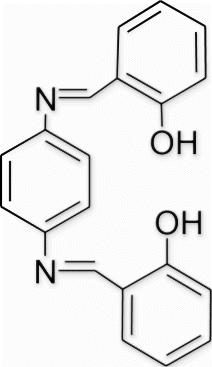
Yield 93%, m.p. 257–258.3 °C, 1H NMR (400 MHz, CDCl3) δH: 6.68–7.12 (m, 8H, arom), 7.41–7.49 (m, 4H, arom), 8.41 (s, 2H, 2N=CH), 9.78 (s, 2H, 2OH); 13C NMR (100 MHz, CDCl3) δc: 115.1, 115.6, 115.7, 121.7, 121.9, 129.8, 130.4, 131.9, 158.9; IR (KBr) νmax: 3533.76 (−OH), 1643.45 (−CH=N−) cm−1; MS, m/z 316 (M+); Anal. Calcd (%) for C20H16N2O2: C, 75.93; H, 5.10; N, 8.86. Found C, 75.92; H, 5.13; N, 8.89.
3b(8). N1,N4 -bis(4-methylbenzylidene)benzene-1,4-diamine (entry 8, table 2)
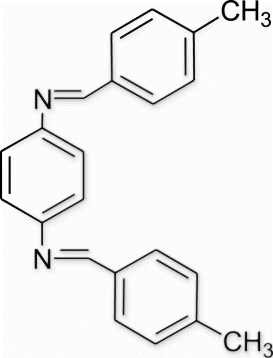
Yield 98%, m.p. 227–228.6 °C, 1H NMR (400 MHz, CDCl3) δH: 2.31 (s, 6H, 2CH3), 7.17−7.21 (m, 8H, arom), 7.93 (s, 4H, arom), 8.41 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 21.1, 121.1, 128.7, 129.4, 135.5, 138.6, 149.2, 159.1; IR (KBr) νmax : 1546.24 (−CH=N−) cm−1; MS, m/z 312.12 (M+); Anal. Calcd (%) for C22H20N2: C, 84.58; H, 6.45; N, 8.97. Found C, 84.60; H, 6.47; N, 8.96.
3b(9). N1,N4 -bis(4-nitrobenzylidene)benzene-1,4-diamine (entry 9, table 2)
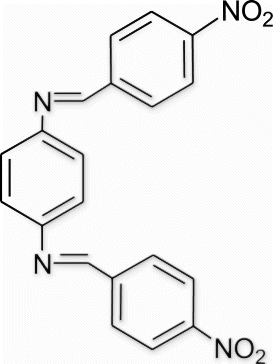
Yield 87%, m.p. 259.1–259.9 °C, 1H NMR (400 MHz, CDCl3) δH: 7.36 (d, 4H, J=8.54, arom), 7.41 (d, 4H, J=8.24, arom), 8.03 (s, 4H, arom), 8.42 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 117.3, 123.5, 129.2, 129.7, 149.9, 158.6, 162.3; IR (KBr) νmax:1607.67 (−CH=N−) cm−1; MS, m/z 374.09 (M+); Anal. Calcd (%) for C20H14N4O4: C, 64.17; H, 3.77; N, 14.97. Found C, 64.15; H, 3.79; N, 14.96.
3b(10). N1,N4 -bis(3-nitrobenzylidene)benzene-1,4-diamine (entry 10, table 2)
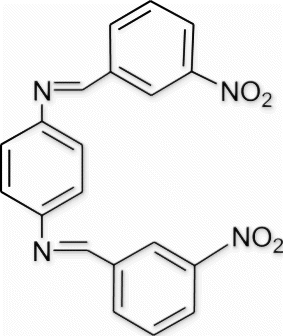
Yield 98%, m.p. 259–260.9 °C, 1H NMR (400 MHz, CDCl3) δH: 7.37 (s, 4H, arom), 7.67–7.73 (m, 6H, arom), 8.01–8.12 (m, 4H, arom), 8.45 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc : 121.7, 127.9, 128.7, 129.3, 130.5, 131.4, 136.2, 149.7, 160.6; IR (KBr) νmax: 1607.67 (−CH=N−) cm−1; MS, m/z 374.09 (M+); Anal. Calcd (%) for C20H14N4O4: C, 64.17; H, 3.77; N, 14.97. Found C, 64.19; H, 3.76; N, 14.96.
3b(11). N1,N4 -diethylidenebenzene-1,4-diamine (entry 11, table 2)
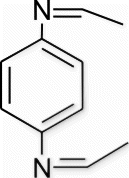
Yield 87%, m.p. 187.1–188.9 °C, 1H NMR (400 MHz, CDCl3) δH: 1.12 (d, 6H, J=8.24, 2CH3), 7.28 (d, 4H, J=7.24, arom), 8.43 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 16.2, 127.8, 128.7, 149.7, 160.3; IR (KBr) νmax: 1643.28 (−CH=N−) cm−1; MS, m/z 161.10 (M+); Anal. Calcd (%) for C10H12N2: C, 74.97; H, 7.55; N, 17.48. Found C, 74.96; H, 7.56; N, 17.46.
3b(12). N1,N4 -dibutylidenebenzene-1,4-diamine (entry 12, table 2)
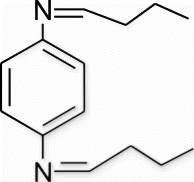
Yield 88%, m.p. 183.7–185.1 °C, 1H NMR (400 MHz, CDCl3) δH: 1.13–1.17 (m, 6H, 2CH3), 1.25–2.04 (m, 8H, 4CH2), 7.32 (d, 4H, J=7.24, arom), 8.41 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 16.1, 27.5, 28.7, 127.6, 128.3, 160.3; IR (KBr) νmax: 1637.91 (−CH=N−) cm−1; MS, m/z 217.2 (M+); Anal. Calcd (%) for C14H20N2: C, 77.73; H, 9.32; N, 12.95. Found C, 77.76; H, 9.33; N, 12.93.
3b(13). N1,N4 -dipentylidenebenzene-1,4-diamine (entry 13, table 2)
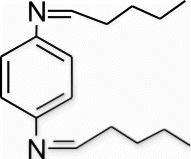
Yield 88%, m.p. 156.9–158.3 °C, 1H NMR (400 MHz, CDCl3) δH: 1.03–1.11 (m, 6H, 2CH3), 1.22–1.94 (m, 8H, 4CH2), 2.21–2.37 (m, 4H, 2CH2), 7.29 (d, 4H, J=7.24, arom), 8.46 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 16.1. 27.1, 27.3, 28.8, 127.6, 128.1, 160.0; IR (KBr) νmax: 1637.91 (−CH=N−) cm−1; MS, m/z 245.2 (M+); Anal. Calcd (%) for C16H24N2: C, 78.64; H, 9.90; N, 11.46. Found C, 78.66; H, 9.93; N, 11.43.
6(1). N-(phenylimino)methyl)benzylidene)benzenamine (entry 1, table 3)
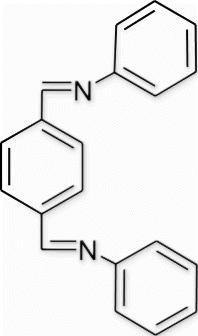
Yield 85%, m.p. 161.7–163.5 °C, 1H NMR (400 MHz, CDCl3) δH: 7.22–7.25 (m, 6H, arom), 7.37–7.43 (m, 4H, arom), 8.00 (s, 4H, arom), 8.49 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 120.6, 126.0, 128.8, 128.9, 138.3, 151.4, 159.1; IR (KBr) νmax: 1577.71 (−CH=N−) cm−1; MS, m/z 285.35 (M+); Anal. Calcd (%) for C20H16N2: C, 84.48; H, 5.67; N, 9.85. Found C, 84.47; H, 5.69; N, 9.86.
6(2). N-(4-chlorophenylimino)methyl)benzylidene)-4-chlorobenzenamine (entry 2, table 3)
Yield 90%, m.p. 175.9–178.3 °C, 1H NMR (400 MHz, CDCl3) δH: 7.19 (d, 4H, J=8.72, arom), 7.36 (d, 4H, J=8.72, arom), 8.00 (s, 4H, arom), 8.48 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 121.7, 129.2, 129.5, 132.3, 138.3, 150.3, 159.4; IR (KBr) νmax: 1579.84 (−CH=N−) cm−1; MS, m/z 352.05 (M+); Anal. Calcd (%) for C20H14Cl2N2: C, 68.00; H, 3.99; N, 7.93. Found C, 68.01; H, 4.01; N, 7.95.
6(3). N-(2-chlorophenylimino)methyl)benzylidene)-2-chlorobenzenamine (entry 3, table 3)
Yield 91%, m.p. 174.3–175.9 °C, 1H NMR (400 MHz, CDCl3) δH: 7.21–7.41 (m, 8H, arom), 8.00 (s, 4H, arom), 8.49 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 121.7, 128.5, 128.9, 129.2, 129.5, 132.3, 138.2, 150.1, 159.4; IR (KBr) νmax: 1579.84 (−CH=N−) cm−1; MS, m/z 352.05 (M+); Anal. Calcd (%) for C20H14Cl2N2: C, 68.00; H, 3.99; N, 7.93. Found C, 68.02; H, 3.97; N, 7.95.
6(4). N-(4-nitrophenylimino)methyl)benzylidene)-4-nitrobenzenamine (entry 4, table 3)
Yield 78%, m.p. 225–226.7 °C, 1H NMR (400 MHz, CDCl3) δH: 7.41 (d, 4H, J=8.24, arom), 7.49 (d, 4H, J=8.72, arom), 8.00 (s, 4H, arom), 8.48 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 122.3, 129.2, 129.7, 131.9, 139.1, 151.3, 159.4; IR (KBr) νmax: 1569.21 (−CH=N−) cm−1; MS, m/z 374.09 (M+); Anal. Calcd (%) for C20H14N4O4: C, 64.17; H, 3.77; N, 14.97. Found C, 64.15; H, 3.76; N, 14.95.
6(5). N-(3-nitrophenylimino)methyl)benzylidene)-3-nitrobenzenamine (entry 5, table 3)
Yield 88%, m.p. 224.5–226.1 °C, 1H NMR (400 MHz, CDCl3) δH: 7.46–7.53 (m, 8H, arom), 7.98 (s, 4H, arom), 8.49 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 122.3, 128.6, 128.9, 129.2, 129.7, 131.9, 139.2, 151.3, 159.1; IR (KBr) νmax: 1569.21 (−CH=N−) cm−1; MS, m/z 374.09 (M+); Anal. Calcd (%) for C20H14N4O4: C, 64.17; H, 3.77; N, 14.97. Found C, 64.15; H, 3.76; N, 14.98.
6(6). N-(p-tolylimino)methyl)benzylidene)-4-methylbenzenamine (entry 6, table 3)
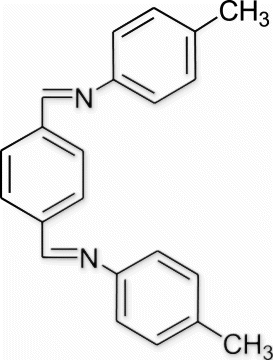
Yield 93%, m.p. 187–189.4 °C, 1H NMR (400 MHz, CDCl3) δH: 2.35 (s, 6H, 2CH3), 7.13–7.17 (m, 8H, arom), 7.96 (s, 4H, arom), 8.48 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 21.1, 121.0, 129.1, 129.9, 136.3, 138.7, 149.2, 158.6; IR (KBr) νmax: 1576.27 (−CH=N−) cm−1; MS, m/z 312.12 (M+); Anal. Calcd (%) for C22H20N2: C, 84.58; H, 6.45; N, 8.97. Found C, 84.54; H, 6.47; N, 8.98.
6(7). N-(o-tolylimino)methyl)benzylidene)-2-methylbenzenamine (entry 7, table 3)
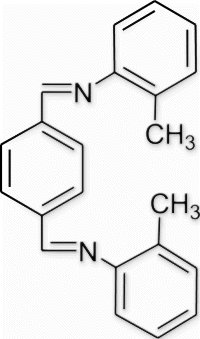
Yield 85%, m.p. 186.7–187.5 °C, 1H NMR (400 MHz, CDCl3) δH: 2.35 (s, 6H, 2CH3), 7.13–7.19 (m, 8H, arom), 7.97 (s, 4H, arom), 8.48 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 21.1, 121.1, 128.7, 129.1, 129.9, 130.3, 136.3, 138.7, 149.2, 158.6; IR (KBr) νmax: 1576.27 (−CH=N−) cm−1; MS, m/z 312.12 (M+); Anal. Calcd (%) for C22H20N2: C, 84.58; H, 6.45; N, 8.97. Found C, 84.55; H, 6.47; N, 8.96.
6(8). N-(((4-methoxyphenylimino)methyl)benzylidene)-4-methoxybenzenamine (entry 8, table 3)
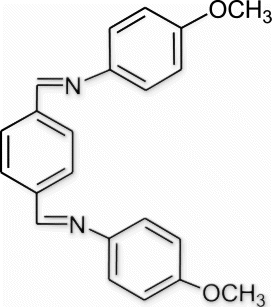
Yield 85%, m.p. 223.5–224.3 °C, 1H NMR (400 MHz, CDCl3) δH: 3.84 (s, 6H, 2OCH3), 6.95 (d, 4H, J=9.16, arom), 7.28 (d, 4H, J=8.68, arom), 7.98 (m, 4H, arom), 8.56 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 55.5, 114.5, 122.4, 128.9, 138.6, 144.6, 157.4, 158.6; IR (KBr) νmax: 1576.98 (−CH=N−) cm−1; MS, m/z 344.15 (M+); Anal. Calcd (%) for C22H20N2O2: C, 76.72; H, 5.85; N, 8.13. Found C, 77.70; H, 5.86; N, 8.10.
6(9). N-(benzylimino)methyl)benzylidene) (phenyl)methanamine (entry 9, table 3)
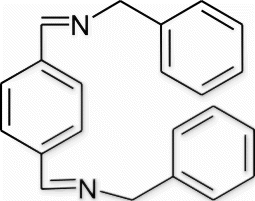
Yield 99%, m.p. 87.8–90.4 °C, 1H NMR (400 MHz, CDCl3) δH: 4.83 (s, 4H, 2CH2), 7.24–7.35 (m, 10H, arom), 7.82 (d, 4H, J=1.6, arom), 8.40 (s, 2H, 2N=CH); 13C NMR (100 MHz, CDCl3) δc: 65.2, 127.1, 128.1, 128.5, 128.6, 138.2, 139.1, 161.4; IR (KBr) νmax: 1577.71 (−CH=N−) cm−1; MS, m/z 313.16 (M+); Anal. Calcd (%) for C22H20N2: C, 84.58; H, 6.45; N, 8.97. Found C, 84.57; H, 6.47; N, 8.98.