Abstract
The synthesis of symmetrical thioethers using organic halides and thiourea in Triton X10 aqueous micelles under basic conditions has been described. Primary alkyl, allyl and benzyl halides can be converted efficiently into symmetrical thioethers in high yields. The entire route is an almost odorless process, and the protocol is applicable to large-scale operation without any problem.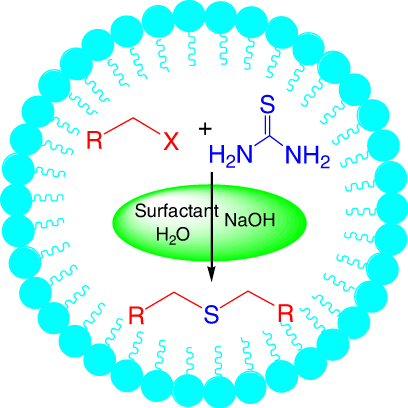
1. Introduction
The development of new, efficient and environmentally benign synthetic protocols for the formation of C–S bonds is an important target in modern organic synthesis Citation1–4. As a result, many recent reports focus on using organic halides and thiols to form C–S bonds Citation5–8; however, the use of highly volatile and foul-smelling thiols leads to serious environmental and safety problems. Recently, it has been found that thiols can be replaced with odorless, non-toxic thiourea as a sulfur source for the formation of C–S bonds Citation9–11.
Water is generally considered as an ideal, “green” solvent for organic transformations based on cost, safety, and environmental impacts. As a result, designing organic reactions in water has become one of the most attractive areas in Green Chemistry Citation12 Citation13. The poor solubility of many reactants in water, however, is the main obstacle in the use of water as a reaction medium, as it may inhibit reactions due to phase separation and inefficient mixing of reactants Citation14. To solve this problem, surfactants are added to form aqueous micelles, which can absorb reactants into a microheterogeneous system Citation15. This can change the reactants’ physical properties, quantum efficiencies and reactivities Citation16, thus accelerating many organic reactions that would be relatively slower in aqueous medium. This is referred to as “micellar catalysis” Citation15–18.
As a part of our interest in using aqueous micelles as a reaction medium, the synthesis of symmetrical thioethers, which is often performed in hot alcohol using organic halides and toxic alkali metal sulfides in industry, has been carried out in Triton X10 (TX10) aqueous micelles using organic halides and thiourea under basic conditions. To the best of our knowledge, there is no report about the synthesis of symmetrical thioethers using organic halides and thiourea in water.
2. Results and discussion
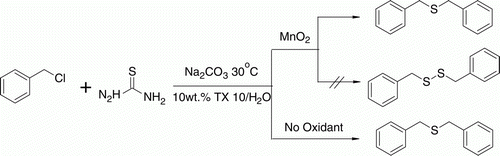
Initially, we wanted to perform the reaction of benzyl chloride and thiourea to produce benzyl disulfide in 10 wt% TX10/H2O using MnO2 as an oxidant. Surprisingly, the reaction failed to provide the desired product and instead yielded benzyl thioether as the final product. Benzyl thioether was also obtained in absence of oxidant (). The reaction of benzyl chloride and thiourea was therefore selected as a model reaction to optimize the reaction conditions for the synthesis of benzyl thioether.
As shown in , use of NaOH could provide a much better yield than use of Na2CO3. The strongly basic aqueous medium could enhance the reaction rate by promoting the formation of the corresponding S-alkylisothiouronium salt (, Entries 1–3) Citation9. After screening different surfactants, sodium dodecyl sulfate (SDS) provided a lower yield than TX10 and CTAB (, Entries 3–5). Presumably, the abstraction of an electron from OH− by S-alkylimidothiocarbamate to form the S-alkylisothiouronium salt was impeded by the anionic micelle-forming agent SDS Citation9 Citation15. TX10 is a cheap, non-toxic surfactant that is widely used in industry as the emulsifier and detergent, so further studies of this reaction were continued with TX10 aqueous micelles.
Table 1. Optimization of the reaction conditions.
To our surprise, the reaction yield of benzyl chloride with thiourea in water was quite high (63%) (, Entry 6). Interestingly, when n-heptyl iodide instead of benzyl chloride was employed under similar conditions, some differences were found. An excellent yield of 90% was obtained in 10 wt% TX10 aqueous micelles, while only a trace amount of desired product was obtained when the reaction was run “on water” (, Entries 7 and 8). These results suggested that the success of these reactions run in the absence of a surfactant is dependent on the substrates. The reason for this could be explained that benzyl chloride had higher reactivity that n-heptyl iodide, so the reaction of thiourea and benzyl chloride could occur, even “on water”.
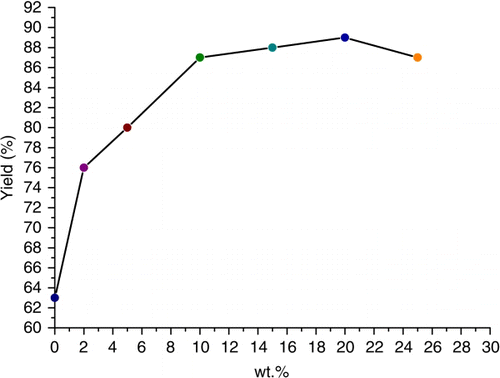
The influence of TX10 concentration was also investigated (). The reaction yields increased with TX10 concentration due to the enlargement of the interfacial area and lower mass transfer resistance Citation15 Citation19. A satisfactory yield (87%) was obtained with 10 wt% TX10 aqueous micelles and no significant change in yield was observed at higher surfactant concentration. Due to the huge interfacial area in micelles, organic halides could efficiently come into contact with thiourea and the micelle droplets formed by TX10 were hydrophobic enough to exclude water molecules Citation5, making it easier to form the S-alkylisothiouronium salt. Thus, the reaction occurred easily within a micelle, which functions as a micro- or nano-reactor.
Figure 1. The influence of TX10's concentration in aqueous micelles on the reaction. Reaction condition: benzyl chloride 2 mmol, thiourea 2 mmol, NaOH 3 mmol, reaction medium 5 mL, 30 °C, 2 h.
With the optimized conditions in hand, the reactions of various organic halides with thiourea were performed to ascertain the generality and scope of the protocol. Benzyl halides and allyl halides were easily transformed into corresponding thioethers with good to excellent yields at 30 °C, but 4-nitrobenzyl chloride needed a longer reaction time and a higher reaction temperature (, Entries 1–7). Primary alkyl halides were also reacted using the protocol by simply prolonging reaction time and increasing temperature to 50 °C. The short chain primary alkyl halides were more reactive substrates compared with those containing long chain (, Entries 8–14), because the reactions of primary alkyl halides and thiourea were bimolecular nucleophilic substitution (SN2) in which less steric hindrance was beneficial to the reaction rate. However, secondary alkyl halides and aryl halides failed to react with thiourea to produce S-alkylisothiouronium salts, owing to their steric hindrance effects and electronic effects, respectively (, Entries 15–19). In order to show the possibility for large-scale operations, we also scaled up the model reaction to 50 mmol, and the reaction proceeded well with an 89% yield of the desired product (, Entry 20).
Table 2. Synthesis of symmetrical thioethers in TX10 aqueous micelles.
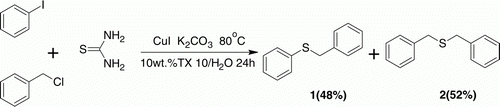
In addition, we also focused on investigating the amount of NaOH to further optimize the reaction conditions, making the protocol more environmentally friendly. Take the reaction of benzyl chloride (2 mmol) and thiourea (2 mmol), as an example, 3.0, 1.0, 0.5, and 0.1 mmol of NaOH were used in the reaction respectively. The corresponding yields were 87, 89, 84, and 63%, indicating that the amount of NaOH could be reduced to 0.5 mmol without significant change in yield. Initial attempts were also made to synthesize an unsymmetrical thioether using iodobenzene, benzyl chloride, and thiourea catalyzed by CuI (20 mol%) in TX10 aqueous micelles. Two thioether products (1:2=48:52) were obtained, indicating the high reactivity of thiourea with benzyl chloride in aqueous micelles ().
3. Experimental
3.1. Materials
Triton X10 (Polyoxyethylene Citation10 octylphenyl ether, TX10) was purchased from the petrochemical plant of Jiangsu Haian. Alkyl bromides were obtained from the corresponding alcohols. All other chemicals (AR grade) were commercially available and used without further purification.
3.2. Typical procedure for the reaction of benzyl chloride and thiourea in TX10 aqueous micelles
To a solution of 10 wt% TX10 aqueous micelles (5 mL) were added benzyl chloride (2 mmol), thiourea (2 mmol), and NaOH (3 mmol) at 30 °C. After consumption of benzyl chloride, which was monitored by gas chromatograph (GC), the reaction mixture was extracted with petroleum ether (5 mL, for three times). The organic layer was collected, dried, and concentrated under reduced pressure to yield the crude product, which was further purified by flash column chromatography on silica gel (petroleum ether). Other symmetrical thioethers were synthesized using a similar procedure.
3.3. Characterization
1H-NMR spectra were recorded on Bruker DRX500 (300 MHz). GC analyses were performed by HP4890 Gas Chromatograph equipped with a flame ionization detector (FID) detector using an Agilent Technologies HP-5 column (15 m×0.530 mm) and a timed program beginning with 1 min at 70 °C followed by 20 °C min−1 ramp to 260 °C then 20 min at this temperature. Gas chromatography-mass spectrometry (GC-MS) data were obtained by using a Saturn2000 GC/MS series. HP 5890 GC was equipped with a CPSTIL-8CB mass selective detector. Mass spectra in the electron impact mode were generated at 70 eV and scan mode in the range of 40–250 amu. Elemental analyses were performed on a Yanagimoto MT3CHN recorder. All products were known compounds identified by comparing their mass spectra with those contained in the mass spectrometer data system library and previously published literature.
The selected data for benzyl thioether (, Entry 1). White solid; mp 45–47 °C; GC-MS [M+] = 213.90; 1H-NMR (300 MHz, CDCl3, δ): 3.67 (s, 4 H), 7.28–7.41 (m, 10 H); Anal. Calcd for C14H14S: C, 78.46; H, 6.58. Found: C, 78.42; H, 6.50.
4. Conclusion
In conclusion, we have described a pronounced catalytic effect of TX10 aqueous micelles for the synthesis of symmetrical thioethers using organic halides and thiourea under basic conditions. The protocol can be applied for large-scale operations easily due to its simple work-up procedure and the elimination of metal. In addition, the use of non-toxic, odorless thiourea instead of thiols or alkali metal sulfides makes the protocol more eco-friendly.
Acknowledgements
We thank Analysis and Testing Center, Nanjing University of Science and Technology for support of this work.
References
- Azizi , N. ; Aryanasab , F. ; Torkiyan , L. ; Ziyaei , A. ; Saidi , M.R. J. Org. Chem . 2006 , 71 , 3634 – 3635 .
- Sreedhar , B. ; Reddy , P.S. ; Reddy , M.A. Synthesis . 2009 , 10 , 1732 – 1738 .
- Paradies , J. Synthesis . 2010 , 947 – 952 .
- Iimura , S. ; Manabe , K. ; Kobayashi , S. Org. Lett . 2003 , 5 , 101 – 103 .
- Manabe , K. ; Iimura , S. ; Sun , X.M. J. Am. Chem. Soc . 2002 , 124 , 11971 – 11978 .
- Miller , K.J. ; Abu-Omar , M.M. Eur. J. Org. Chem . 2003 , 1294 – 1299 .
- Alvaro , E. ; Hartwig , J.F. J. Am. Chem. Soc . 2009 , 131 , 7858 – 7868 .
- Fernández-Rodríguez , M.A. ; Hartwig , J.F. J. Org. Chem . 2009 , 74 , 1663 – 1672 .
- Firouzabadi , H. ; Iranpoor , N. ; Gholinejad , M. Adv. Synth. Catal . 2010 , 352 , 119 – 124 .
- Firouzabadi , H. ; Iranpoor , N. ; Abbasi , M. Adv. Synth. Catal . 2009 , 351 , 755 – 766 .
- Firouzabadi , H. ; Iranpoor , N. ; Abbasi , M. Tetrahedron . 2009 , 65 , 5293 – 5301 .
- Anastas , P.T. ; Warner , J.C. Green Chemistry: Theory and Practice ; Oxford , , UK : Oxford University Press , 1998 .
- Chanda , A. ; Fokin , V.V. Chem. Rev . 2009 , 109 , 725 – 748 .
- Lindstrom , U.M. Chem. Rev . 2002 , 102 , 2751 – 2772 .
- Dwars , T. ; Paetzold , E. ; Oehme , G. Angew. Chem. Int. Ed . 2005 , 44 , 7174 – 7199 .
- Tascioglu , S. Tetrahedron . 1996 , 52 , 11113 – 11152 .
- Cordes , E.H. ; Dunlap , R.B. Acc. Chem. Res . 1969 , 2 , 329 – 337 .
- Stavber , G. ; Zupan , M. ; Stavber , S. Synlett . 2009 , 589 – 594 .
- Jiang , J.Z. ; Cai , C. J. Colloid Interface Sci . 2006 , 299 , 938 – 943 .