Abstract
A simple, fast, and highly efficient protocol has been developed for the synthesis of 9,10-dihydro-anthracene-9,10-α,β-succinic imide derivatives by simple mixing and grinding of 9,10-dihydro-anthracene-9,10-α,β-succinic anhydride with various aliphatic, aromatic amines, and hydrazines. This catalyst and solvent-free green approach provided the condensation products within few minutes at room temperature in quantitative yield.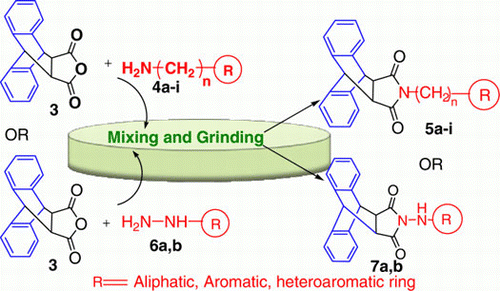
Introduction
Solvent- and catalyst-free green synthesis occupies a unique segment in chemical space. With the growing awareness in academic, research, and industrial laboratories for sustainable development, the research community is under increasing pressure to alter current working practices and to find greener alternatives Citation1 Citation2.Therefore, it is not surprising and is reflected in an exponential increase in the productivity of scientific papers, books Citation3 Citation4, and reviews Citation5 Citation6 related to the use of green chemistry. In recent years, researchers have applied green technology as a tool in order to reduce reaction time, diminish side products, increase yield, and simplify the course of reactions for combinatorial chemistry Citation7. This solvent- and catalyst-free synthetic technology has shown broad applications as a very efficient way to develop the course of many organic reactions; so as a result, it has been promoted worldwide by an increasing number of research groups because it provides transformations that are environmentally benign Citation8 Citation9.
In the past decade, aromatic compounds bearing imide moiety are significant organic species Citation10–16, which promote a wide range of applications as optoelectronic devices Citation17, chemical sensors Citation18, supramolecular assemblies Citation19, and organic semiconductors Citation20. Along with the photophysical and chemical properties, organic imides and diimides also play significant role in pharmacology. Imides possess wide range of biological activities such as anti-inflammatory Citation21–24, anticancer Citation25 Citation26, antimicrobial Citation27, DNA binding, and apoptotic-inducing activities Citation28, etc. They also act as selective α1d-adrenergic receptor antagonists which modulate intercellular biochemical processes in response to changes in extracellular concentrations of the neurotransmitter norepinephrine and the circulating hormone epinephrine, leading to widespread physiological actions that make them attractive targets for drug discovery Citation29 Citation30. Well-known drug thalidomide a synthetic glutamic acid derivative, bearing two imide moieties, has interesting pharmacological properties Citation31.
Due to the immense importance of imide molecules, it is necessary to review the synthesis of imide derivatives. Various methods are reported by several research groups in literature for synthesis of imide derivatives Citation32–35. Current interest includes developing new imide molecules to broaden the imides applications and further understand the relationship between molecular structure and properties. It is necessary to develop novel synthetic methodology to synthesize novel types of aromatic imides with π-conjugated cores. N-substituted imide bond formation frequently requires the low-yielding condensation of an amine with a free anhydride at high temperature for long time.
As part of our ongoing research work on the development of new molecules of biological interest using green approaches in organic synthesis Citation36 Citation37, herein we report our results on near absolute green protocol for the synthesis of N-substituted 9,10-dihydro-anthracene-9,10-α,β-succiniimides by condensation of various amines and hydrazines with 9,10-dihydro-anthracene-9,10-α,β-succinic anhydride under solvent- and catalyst-free conditions.
Results and discussion
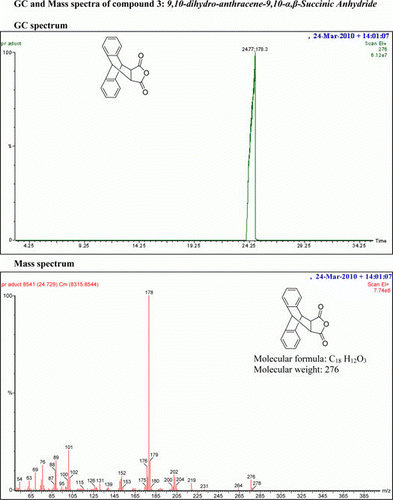
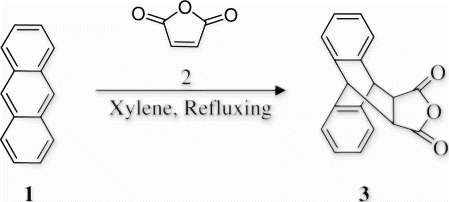
9,10-Dihydro-anthracene-9,10-α,β-succinic anhydride 3 has been synthesized by reaction of anthracene 1 and maleic anhydride 2 () under conventional heating Citation38 in 85% yield, (mp 261–263°C).
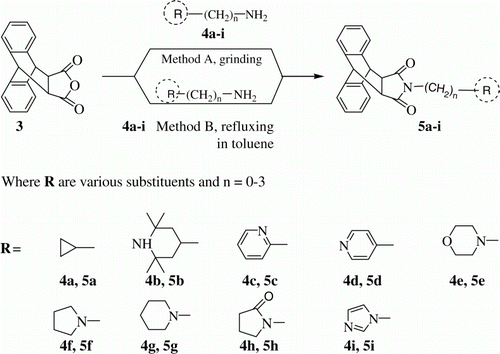
Reaction between 9,10-dihydroanthracene-9,10-α,β-succinic anhydride and various amines: As a prelude to this objective, we carried out the reaction between liquid–solid reactants. Thus, equimolar amounts of 9,10-dihydro-anthracene-9,10-α,β-succinic anhydride (3) and cyclopropanamine (4a) having n=0, (Method A, ) were taken in a petri dish and mixed thoroughly with spatula followed by vigorously grinding for 2 minutes and then the mixture was allowed to stand in a desiccator for 5 minutes. The progress of the reaction was monitored by thin-layer chromatography (TLC) over silica gel using ethyl acetate/methanol (19:1) as mobile phase. During this period, complete conversion from the reactants to imide took place in this atom economy reaction.
After completion of the reaction, product was purified via washing with diethyl ether followed by simple crystallization from methanol to obtain product 5a in good yield. The purity of the product 5a was good enough for spectroscopic analysis. Compound 5a was fully characterized by IR, 1H NMR, GC-MS spectroscopic data, and elemental analysis.
For further verification, this reaction was (Method B, ) performed under conventional heating. Equimolar amounts of 9,10-dihydro-anthracene-9,10-α,β-succinic anhydride (3) and cyclopropanamine (4a) (Method B, ) were dissolved in toluene and refluxed on sand bath. Reaction progress was monitored by TLC over silica gel using ethyl acetate: methanol (19:1) as mobile phase. After completion of the reaction in 2 hour, product was purified by washing with diethyl ether followed by crystallization from methanol to give pure product 5a. Compound 5a was fully characterized by IR, 1H NMR, GC-MS spectroscopic data, and elemental analysis. Spectroscopic data of compound 5a reveals that the product obtained via Method A and method B was pure and same.
After a successful attempt with amine in which value of n is 0, we further explored the reaction of 9,10-dihydro-anthracene-9,10-α,β-succinic anhydride (3) with various amines (n=0, 1, 2, 3 and three- to six-membered ring system). A series of product 5a–i have been synthesized via condensation of compound 3 with various amines (4a–i). All the compounds 5a–i were synthesized by following Method A (grinding the equimolar ratio of both reactant, solvent- and catalyst-free condition) and Method B (by refluxing both the reactants in toluene for 2–5 h, conventional method).
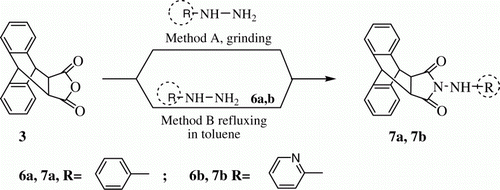
In further exploration of this method, anthracene imides 7a and 7b () have been synthesized by both the methods grinding method and conventional heating method and results are summarized in . This method is also applicable to hydrazine and heterocyclic hydrazine, that is phenylhydrazine and 2-hydrazinopyridine with, 9,10-dihydro-anthracene-9,10-α,β-succinic anhydride. Both the compounds 7a and 7b were characterized by IR, H NMR, GC-MS spectroscopic data, and elemental analysis. Reaction time taken, percentage yield, and melting point (mp) of all the compounds (5a–i, 7a, and 7b) synthesized are mentioned in .
Table 1. Grinding time (in min, Method A), refluxing time (hours, Method B), percentage yield and melting point of anthracene derivatives 5a–i and 7a, 7b.
In the course of our investigations reactions were carried out by fast grinding of stoichiometric molar ratio of both the reactants with a spatula in a petri dish for different time intervals (2–5 min) and allowing the contents to stand for 5 minutes in desiccator. In all these cases, the reaction proceeded smoothly to produce the condensation products in quantitative yield.
Experimental
Melting points were determined on a JSGW apparatus and are uncorrected. IR spectra were recorded using a Perkin–Elmer 1600 FT spectrometer. 1H NMR spectra were recorded on a Bruker WH-500 spectrometer at a ca 5–15% (w/v) solution in DMSO-d 6. GC-MS was recorded on Perkin–Elmer Clarus 500 gas chromatograph where built in MS detector was used. Elemental analysis was carried out on a Vario EL III elementor. TLC was performed on silica gel G for TLC (Merck) and spots were visualized by iodine vapor or by irradiation with ultraviolet light (short wave length, 254 nm).
General procedure for synthesis of 9,10-dihydro-anthracene-9,10-α,β-succinic imides
Method A
9,10-dihydro-anthracene-9,10-α,β-succinic anhydride (3; 552 mg, 2 mmol) and cyclopropanamine 4a (114 mg, 2 mmol) were mixed together thoroughly and grinded vigorously with a spatula for 2 minutes in a petri dish to form a homogeneous paste and then the mixture was allowed to stand for 5 minutes in a desiccator. Completion of reaction was monitored by TLC over silica gel using ethyl acetate: methanol (19:1) as mobile phase. The solid product so obtained was purified by washing with diethyl ether followed by crystallization from methanol to give pure product 5a in 92% yield.
Method B
9,10-dihydro-anthracene-9,10-α,β-succinic anhydride (3; 276 mg, 1 mmol) and cyclopropanamine 4a (57 mg, 2 mmol) were dissolved in dry tetrahydrofuran and kept on refluxing on sand bath. Reaction progress was monitored by TLC over silica gel using ethyl acetate: methanol (19:1) as mobile phase. After completion of the reaction in 3 hour, reaction contents were cooled in cold water bath. So obtained solid was filtered and this crude product was purified by washing with diethyl ether followed by crystallization from methanol to give pure product 5a in 72% yield. Similarly compounds 5b–i, 7a, and 7b were synthesized.
9,10-Dihydro-anthracene-9,10-α,β-succinic anhydride: (3)
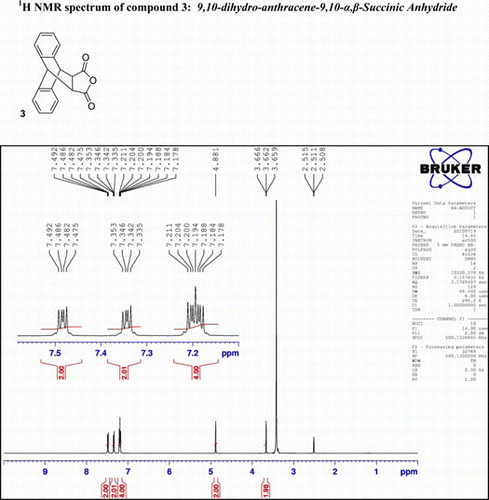
IR (KBr) υmax: 1650 (–CO–O–CO–), 1601, 1470 (Ar) cm−1; 1H NMR (DMSO-d 6, 500 MHz) δ: 3.659–3.666 (t, 2H, 2×CH), 4.881 (s, 2H, 2×CH), 7.178–7.211 (d, 4H, Ar), 7.335–7.353 (q, 2H, Ar), and 7.475–7.492 (q, 2H, Ar). GC-MS: m/z 276 (5%).
N-(Cyclopropyl)-9,10-dihydro-anthracene-9,10-α,β-succinimide, (5a)
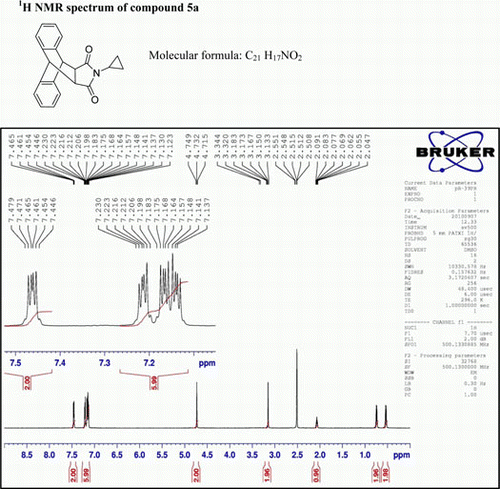
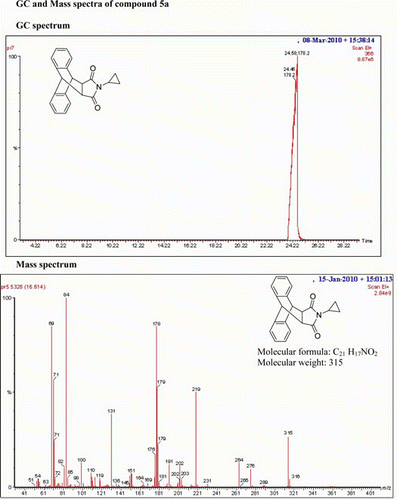
IR (KBr) νmax:1711 (–CO–N–CO–), 1461, 1403 (Ar) cm−1; 1H NMR (DMSO-d 6, 500 MHz) δ: 0.501–0.509 (m, 2H, CH2), 0.521–0.573 (m, 2H, CH2), 2.047–2.091 (m, 1H, CH), 3.133–3.150 (d, 2H, J=8.5 Hz), 4.715–4.732 (d, 2H, J=8.5 Hz), 7.137–7.230 (m, 6H, Ar), 7.446–7.479 (m, 2H, Ar). GC-MS: m/z 315 (M+, 25%). Analysis calculated for C21 H17NO2: C, 80.00; H, 5.39; N, 4.44. Found:C, 79.96; H, 5.38; N, 4.40.
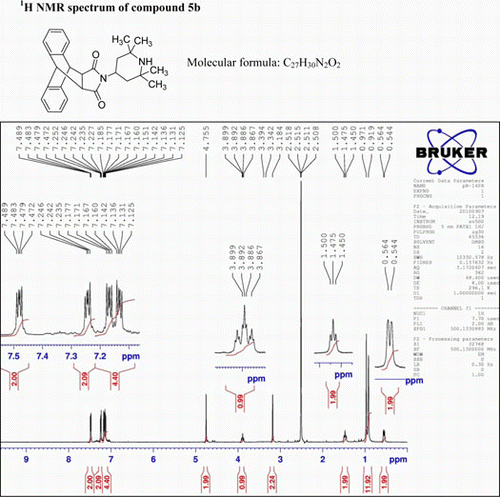
N-(4-(2,2,6,6-tetramethyl)piperadine)-9,10-dihydro anthracene-9,10-α,β-succinimide, (5b)
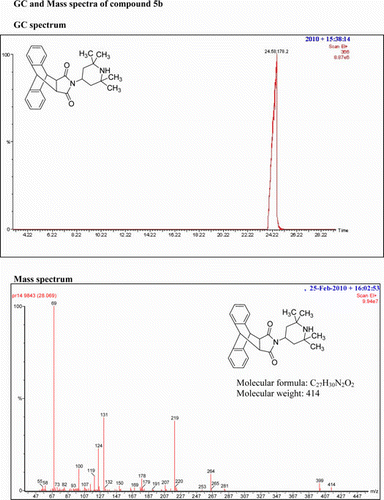
IR (KBr) νmax:1699 (–CO–N–CO–), 1498, 1401 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 0.544–0.564 (d, 2H, CH2), 0.919 (s, 6H, 2×CH3), 0.9711.011 (s, 6H, 4×CH3), 1.450–1.500 (t, 2H, J=12.5 Hz, CH2), 3.184 (s, 2H, 2× CH), 3.67–3.899 (t, 1H, J = 3.5Hz, CH2), 4.755 (s, 2H, 2×CH), 7.125–7.142 (m, 2H, Ar), 7.160–7.185 (m, 2H, Ar), 7.227–7.252 (m, 2H, Ar), 7.465–7.489 (m, 2H, Ar). GC-MS: m/z 414 (M+, 8%). Analysis calculated for C27H30N2O2: C, 78.26; H, 7.24; N, 6.76. Found: C, 78.25; H, 7.24; N, 6.73.
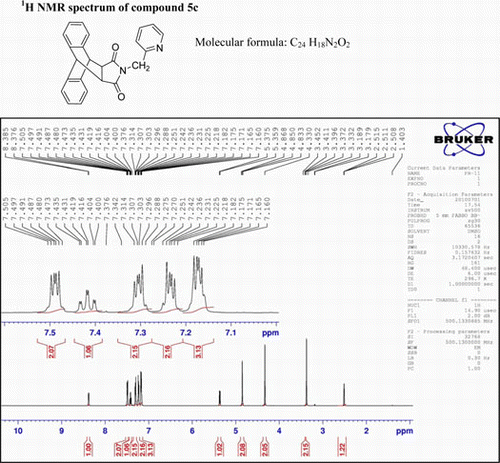
N-(2-Picolyl)-9,10-dihydro-anthracene-9,10-α,β-succinimide, (5c)
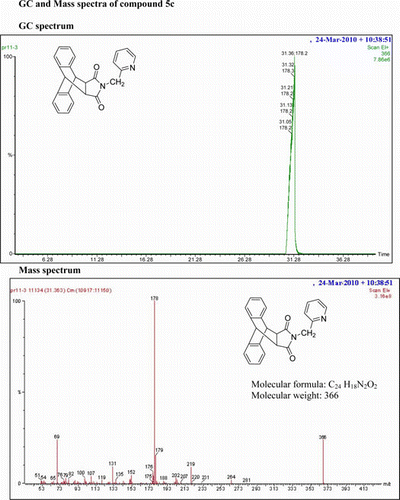
IR (KBr) νmax:1698 (–CO–N–CO–), 1462, 1398 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 3.452 (s, 2H, 2×CH), 4.330 (s, 2H, CH2), 3.868 (s, 2H, 2×CH), 5.359–5.375 (t, 1H, J=8 Hz, py). 7.160–7.182 (m, 3H, 2H of Ar +1H of py), 7.218–7.251 (m, 2H, Ar), 7.288–7.314 (m, 2H, Ar), 7.400–7.435 (m, 1H, py), 7.473–7.505 (m, 2H, Ar) 8.376–8.385 (d, 1H, J=4.5 Hz, py). GC-MS: m/z 366 (M+, 28%). Analysis calculated for C24H18N2O2: C, 78.68; H, 4.92; N, 7.65. Found: C, 78.65; H, 4.91; N, 7.65.
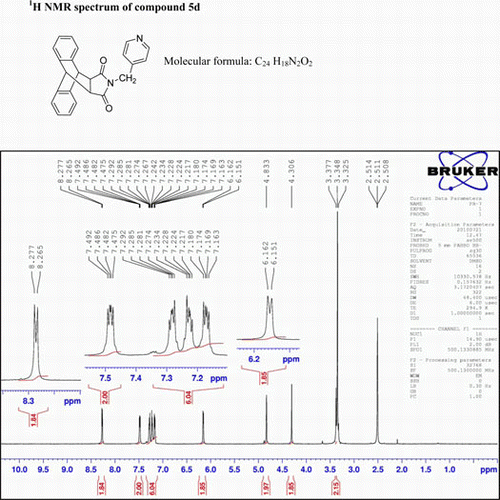
N-(4-Picolyl)-9,10-dihydro-anthracene-9,10-α,β-succinimide: (5d)
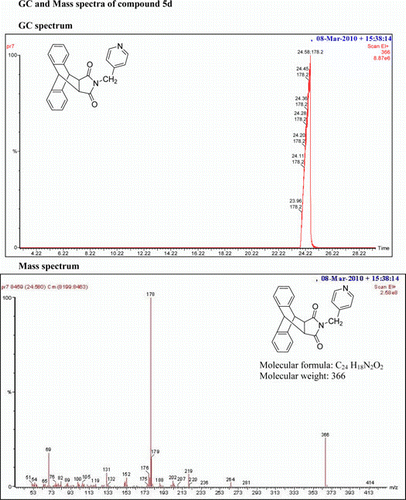
IR(KBr)νmax: 1715 (–CO–N–CO–), 1651 (CO), 1615, 1548, 1319 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 3.377 (s, 2H, 2×CH), 4.306 (s, 2H, CH2), 4.833 (s, 2H, 2×CH), 6.151–6.162 (d, 2H, J=5.5 Hz, py). 7.163–7.180 (m, 2H, Ar), 7.217–7.234 (m, 2H, Ar), 7.274–7.292 (m, 2H, Ar), 7.475–7.492 (m, 2H, Ar), 8.265–8.277 (d, 2H, J=6 Hz, py). GC-MS: m/z 366 (M+, 22%). Analysis calculated for C24 H18N2O2: C, 78.68; H, 4.92; N, 7.65. Found: C, 78.64; H, 4.92; N, 7.64.
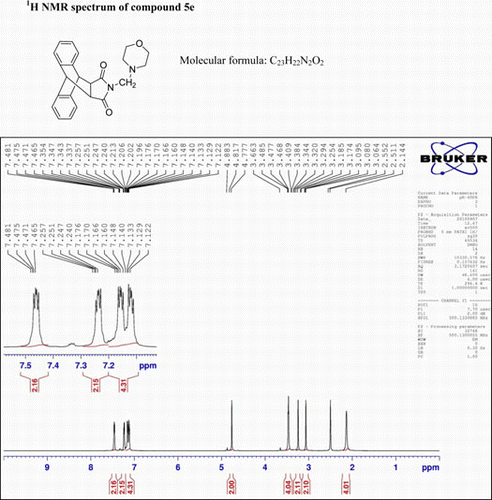
N-(2-Methylmorpholin)-9,10-dihydro-anthracene-9,10-α,β-succinimide: (5e)
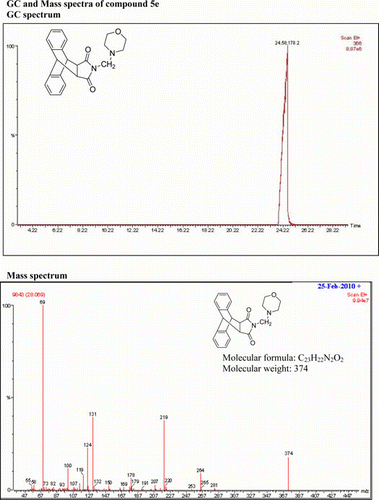
IR (KBr) νmax: 1705(–CO–N–CO–), 1651(CO), 1461, 1438, 1395 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 2.144 (bs, 4H, 2×CH2), 3.254 (s, 2H, CH2), 3.344 (s, 2H, 2×CH), 3.663 (s, 4H, 2×CH2), 4.817(s, 2H, 2×CH), 7.122–7.140 (m, 2H, Ar), 7.160–7.176 (m, 2H, Ar), 7.240–7.257 (m, 2H, Ar), 7.465–7.481 (m, 2H, Ar). GC-MS: m/z 374 (M+, 10%). Analysis calculated for C23H22N2O3: C, 73.79; H, 5.88; N, 7.48. Found: C, 73.76; H, 5.88; N, 7.64.
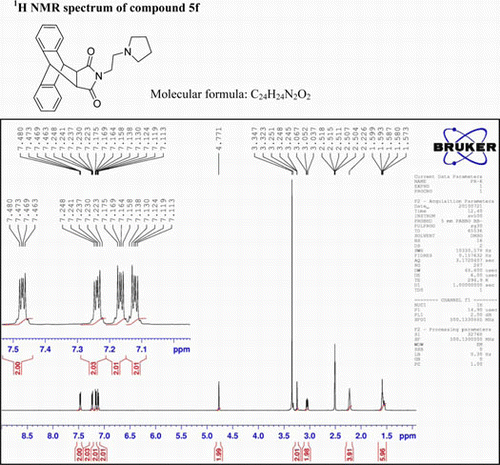
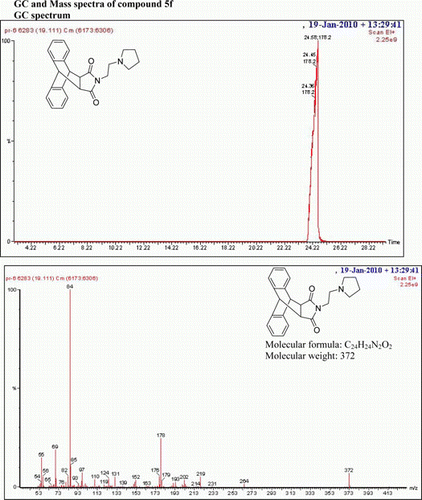
N-((2-Pyrrolidyl)ethyl)-9,10-dihydro-anthracene-9,10-α,β-succinimide: (5f)
IR (KBr) νmax: 1707(–CO–N–CO–), 1646(CO), 1546, 1459, 1319 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 1.538–1.599 (m, 6H, aliphatic), 2.226 (bs, 4H, aliphatic), 3.037–3.067 (t, 2H, CH2), 3.251 (s, 2H, 2×CH), 4.771 (s, 2H, 2×CH), 7.113–7.138 (m, 2H, Ar), 7.158–7.175 (m, 2H, Ar), 7.223–7.248 (m, 2H, Ar), 7.463–7.480 (q, 2H, J=3, J= 5, J=8.5, Ar). GC-MS: m/z 372 (M+, 5%). Analysis calculated for C24 H24N2O2: C, 77.42; H, 6.45; N, 7.52. Found: C, 77.41; H, 6.40; N, 7.52.
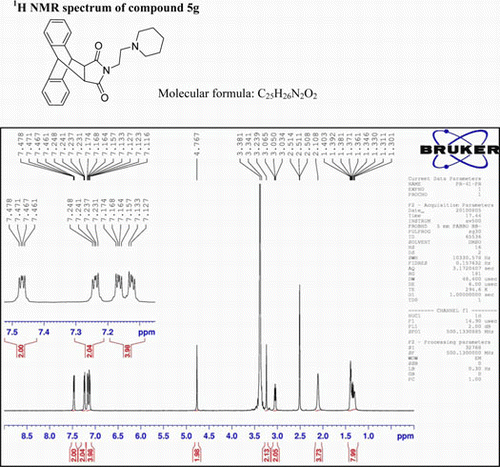
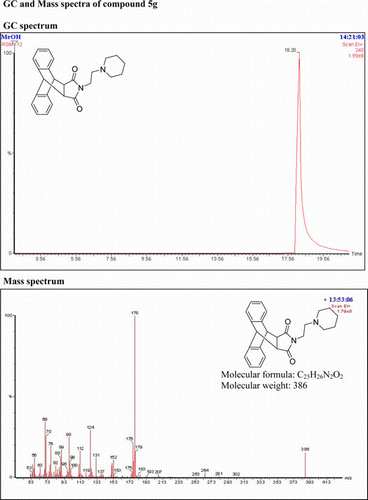
N-(3-(Piperidinyl)ethyl)-9,10-dihydro-anthracene-9,10-α,β-succinimide: (5g)
IR (KBr) νmax: 1702(–CO–N–CO–), 1646(CO), 1457, 1398, 1333 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 1.301–1.403 (m, 8H, aliphatic), 2.108 (bs, 4H, aliphatic), 3.034–3.065 (t, 2H, CH2), 3.239 (s, 2H, 2×CH), 4.767 (s, 2H, 2×CH), 7.116–7.174 (m, 4H, Ar), 7.231–7.248 (m, 2H, Ar), 7.461–7.478 (q, 2H, Ar). GC-MS: m/z 386 (M+, 8%). Analysis calculated for C25H26N2O2: C, 77.72; H, 6.73; N, 7.25. Found: C, 77.70; H, 6.71; N, 7.21.
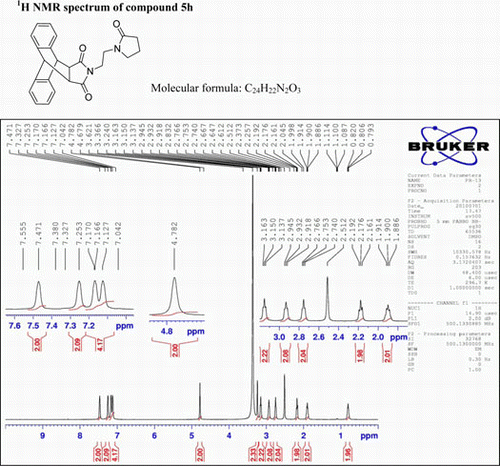
N-(3-(Pyrrolidin-2-one)propyl)-9,10-dihydro-anthracene-9,10-α,β-succinimide: (5h)
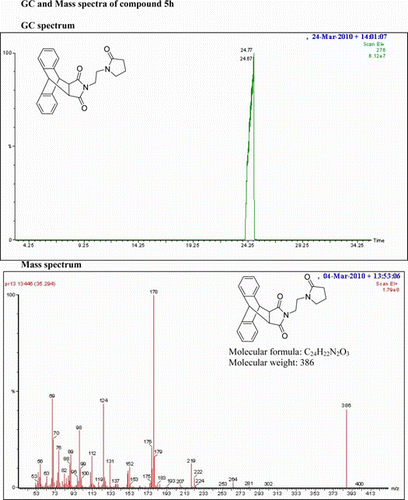
IR (KBr) νmax: 1697(–CO–N–CO–), 1462, 1398 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 0.793–0.820(t, 2H, CH2), 1.886–1.914 (t, 2H, J=7 Hz, CH2), 2.161–2.192(t, 2H, J=7.5 Hz, CH2), 2.740–2.766 (t, 2H, J=6.5 Hz, CH2), 2.918–2.945 (t, 2H, J=6.5 Hz, CH2), 3.137–3.163(t, 2H, J=6.5 Hz, CH2), 3.621 (s, 2H, 2×CH), 4.782 (s, 2H, 2×CH), 7.127–7.170 (m, 4H, Ar), 7.253 (s, 2H, Ar), 7.471 (s, 2H, Ar). GC-MS: m/z 386 (M+, 40%). Analysis calculated for C24H22N2O3: C, 74.61; H, 5.70; N, 7.25. Found: C, 74.59; H, 5.68;N,7.22.
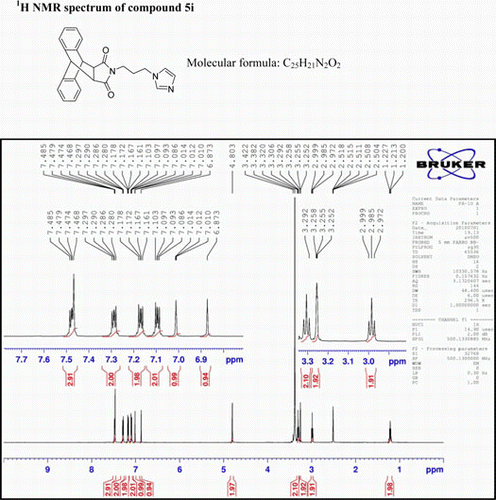
N-[3-(1H-imidazole-1-yl)propyl]-9,10-dihydro-anthracene-9,10-α,β-succinamide: (5i)
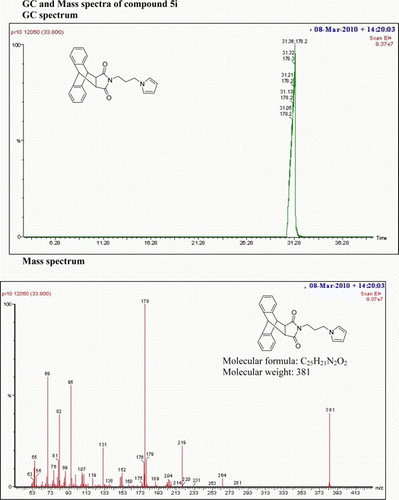
IR(KBr)νmax: 1708(–CO–N–CO–), 1647(>C=N–), 1546 and1459 (Ar) cm−1 1H NMR (500 MHz; DMSO-d 6) δ (ppm): 7.468–7.485 (q, 3H, Ar), 7.280–7.297 (q, 2H, Ar) 7.161–7.178 (q, 2H, Ar), 7.086–7.103(q, 2H, Ar), 7.010–7.014 (t, 1H, Ar), 6.873 (s, 1H, Ar), 4.803 (s, 2H, 2×CH), 3.292–3.320 (t, 2H, 2×CH), 3.252–3.258 (t, 2H, CH2), 2.972–2.999 (t, 2H, CH2), 1.200–1.227 (t, 2H, CH2) GC-MS m/z 383 (M+, 3%). Analysis calculated for C24H21N3O2 C 75.19; H 5.48; N 10.96 Found C 76.20; H 4.26; N 9.16.
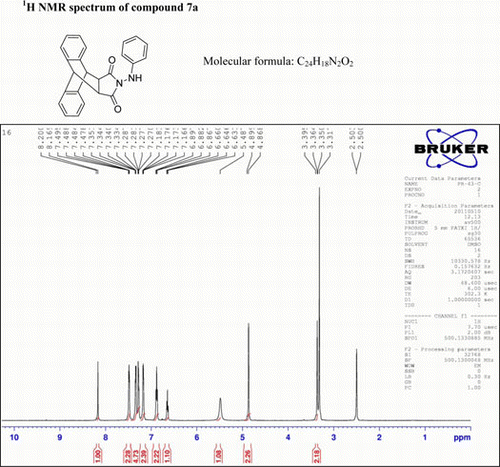
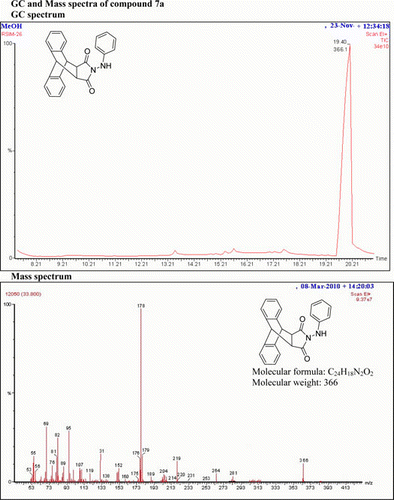
N-(1-Phenylhydrazino)-9,10-dihydro-anthracene-9,10-α,β-succinamide: (7a)
IR (KBr) νmax: 3112 (NH), 1703 (–CO–N–CO–), 1512, 1490, 1396 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 3.395 (s, 2H, 2× CH), 4.868 (s, 2H, 2×CH), 5.483 (bs, 1H, NH, D2O exch.), 6.633–6.660 (m, 1H, Ar), 6.863–6.689( m, 2H, Ar), 7.16–7.18 (m, 2H, Ar), 7.270–7.283 (m, 2H, Ar), 7.34–7.35 (m, 2H, Ar), 7.47–7.49 (m, 2H, Ar), 8.163–8.200 (d, 1H, Ar). GC-MS: m/z 366 (M+, 10%). Analysis calculated for C24H18N2O2: C, 78.26; H, 7.24; N, 6.76. Found: C, 78.25; H, 7.24; N, 6.73.
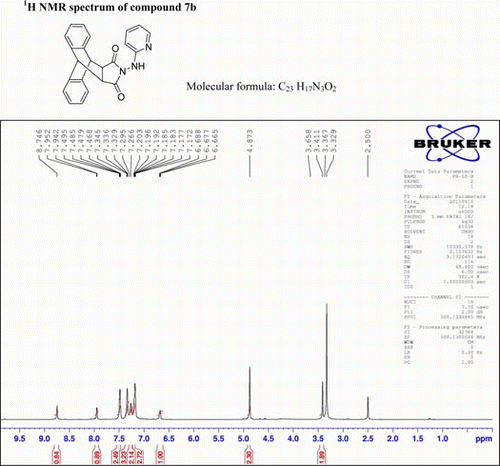
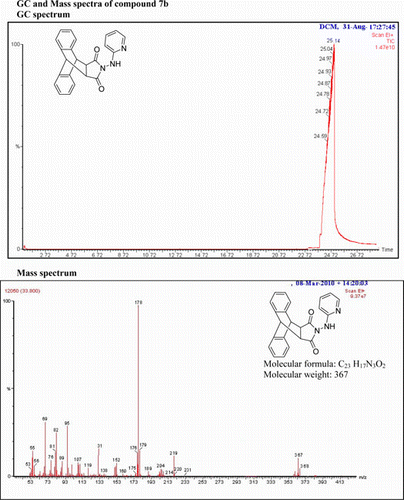
N-(1-(Pyridin-2-yl)hydrazino)-9,10-dihydro-anthracene-9,10-α,β-succinamide: (7b)
IR(KBr)νmax:3098 (NH), 1701 (–CO–N–CO–), 1520, 1495, 1400 (Ar) cm−1. 1H NMR (DMSO-d 6, 500 MHz) δ: 3.658 (s, 2H, 2× CH), 4.873(s, 2H, 2×CH), 6.665–6.688 (t, 1H, Ar), 7.172–7.203(m, 2H, Ar), 7.266–7.295 (d, 2H, Ar), 7.329–7.345 (t, 3H, Ar), 7.468–7.495 (d, 2H, Ar), 7.942–7.952 (d, 1H, Ar), 8.746 (s, 1H, Ar). GC-MS: m/z 367 (M+, 6%). Analysis calculated for C23H17N3O2: C, 75.20; H, 4.63; N, 11.44. Found: C, 75.11; H, 4.57; N, 11.52.
Conclusion
In conclusion, we have demonstrated a simple, atom economy, fast, and highly efficient solvent-free green protocol for synthesis of antharacene imide derivatives. To the best of our knowledge, this green protocol is not known in literature. Further detailed studies on the biological evaluation of these antharacene imide derivatives are in progress.
Acknowledgements
Ms. Reshma Rani and Surbhi Arya are thankful to CSIR, New Delhi, for financial assistance.
References
- Baird , C. Environmental Chemistry ; W.H. Freeman and Company : New York , 1999 .
- Anastas , P. , Heine , L.G. and Williamson , T.C. 2000 . Green Chemical Syntheses and Processes , New York : Oxford University Press .
- Anastas , P.T. : Warner , J.C. In Green Chemistry: Theory and Practice ; Anastas , P.T. Warner , J.C. Oxford : Oxford University Press , p. 60 1998 .
- Lancaster , M. In Handbook of Green Chemistry and Technology ; Clark , J.H. , Macquarrie , D.J. , Blackwell Publishing : Abingdon , 2002 .
- Fischmeister , C. ; Doucet , H. Green Chem . 2011 , 13 , 741 .
- Frontana-Uribe , B.A. ; Little , R.D. ; Ibanez , J.G. ; Palma , A. ; Vasquez-Medrano , R. Green Chem . 2010 , 12 , 2099 .
- Anastas , P.T. ; Bartlett , L.B. ; Kirchhoff , M.M. ; Williamson , T.C. Catal. Today 2000 , 55 , 11 .
- Bruckmann , A. ; Krebs , A. ; Bolm , C. Green Chem . 2008 , 10 , 1131 .
- Horváth , I.T. Green Chem . 2008 , 10 , 1024 .
- Weitz , R. T. ; Amsharov , K. ; Zschieschang , U. ; Villas , E. B. ; Goswami , D. K. ; Burghard , M. ; Dosch , H. ; Jansen , M. ; Kern , K. ; Klauk , H. J. Am. Chem. Soc . 2008 , 130 , 4637 .
- Ling , M.-M. ; Erk , P. ; Gomez , M. ; Koenemann , M. ; Locklin , J. ; Bao . Z. Adv. Mater . 2007 , 19 , 1123 .
- Gao , X. ; Wang , Y. ; Yang , X. ; Liu , Y. ; Qiu , W. ; Wu , W. ; Zhang , H. ; Qi , T. ; Liu , Y. ; Lu , K. ; Du , C. ; Shuai , Z. ; Yu , G. ; Zhu , D. Adv. Mater 2007 , 19 , 3037 .
- Kucheryavy , P. ; Li , G. ; Vyas , S. ; Hadad , C. ; Glusac , K.D. J. Phys. Chem. A , 2009 , 113 , 6453 .
- Rogers , J.E. ; Weiss , S.J. ; Kelly , L.A. J. Am. Chem. Soc . 2000 , 122 , 427 – 436 .
- Fuller , M.J. ; Sinks , L.E. ; Rybtchinski , B. ; Giaimo , J.M. ; Li , X. ; Wasielewski , M. R. J. Phys. Chem. A 2005 , 109 , 970 .
- Lukas , A.S. ; Zhao , Y. ; Miller , S.E. ; Wasielewski , M. R. J. Phys. Chem. B 2002 , 106 , 1299 .
- Gao , X. ; Di , C. ; Hu , Y. ; Yang , X. ; Fan , H. ; Zhang , F. ; Liu , Y. ; Li , H. ; Zhu , D. J. Am. Chem. Soc . 2010 , 132 , 3697 .
- Mukhopadhyay , P. ; Iwashita , Y. ; Shirakawa , M. ; Kawano , S. ; Fujita , N. ; Shinkai , S. Angew. Chem. Int. Ed . 2006 , 45 , 1592 .
- You , C.-C. ; Würthner , F. J. Am. Chem. Soc . 2003 , 125 , 9716 .
- Schmidt , R. ; Oh , J.H. ; Sun , Y.-S. ; Deppisch , M. ; Krause , A.-M. ; Radacki , K. ; Braunschweig , H. ; Konemann , M. ; Erk , P. ; Bao , Z. ; Wurthner , F. J. Am. Chem. Soc . 2009 , 131 , 6215 .
- Alexendre , M.S. ; Int. Immunopharmacol . 2005 , 5 , 485 .
- Sondhi , S.M. ; Rani , R. ; Roy , P. ; Agrawal , S.K. ; Saxena , A.K. Bioorg. Med. Chem. Lett . 2009 , 46 , 1369 .
- Huang , H.-S. Chem. Abstr . 2002 , 136 , 304069. US6372785 .
- Sondhi , S.M. ; Rani , R. ; Diwvedi , A.D. ; Roy , P. J. Heterocycl. Chem . 2009 , 46 , 1369 .
- Hénon , H. ; Messaoudi , S. ; Anizon , F. ; Aboab , B. ; Kucharczyk , N. ; Léonce , S. ; Golsteyn , R.M. ; Pfeiffer , B. ; Prudhomme , M. Eur. J. Pharmacol . 2007 , 554 , 106 .
- Laronze , M. ; Boisbrun , M. ; Le′once , S. ; Pfeiffer , B. ; Renard , P. ; Lozach , O. ; Meijer , L. ; Lansiaux , A. ; Bailly , C. ; Sapi , J. ; Laronzea , J.Y. Bioorg. Med. Chem . 2005 , 13 , 2263 .
- Anizon , F. ; Belin , L. ; Moreau , P. ; Sancelme , M. ; Voldoire , A. ; Prudhomme , M. ; Ollier , M. ; Severe , D. ; Riou , J.F. ; Bailly , C. ; Fabbro , D. ; Thomas , M. J. Med. Chem . 1997 , 40 , 3456 .
- Abdel-Aziz , A.A.-M. Eur. J. Med. Chem . 2007 , 42 , 614 .
- Konkel , M.J. ; Wetzel , J.M. ; Cahir , M. ; Craig , D.A. ; Noble , S.A. ; Gluchowski , C.J. Med. Chem . 2005 , 48 , 3076 .
- Ruffolo , R.R. Jr ; Bondinell , W.E. ; Hieble , J.P.R . J. Med. Chem . 1995 , 38 , 3681 .
- Melchert , M. ; List , A. ; Int. J. Biochem. Cell Biol . 2007 , 39 , 1489 .
- Kometani , T. ; Fitz , T. ; Watt , D.S. Tetrahedron Lett . 1986 , 27 , 919 .
- Stiz , D.S. ; Souza , M.M. ; Golin , V. ; Neto , R.A.S. ; Correa , R. ; Nunes , R.J. ; Yunes , R.A. ; Cechinel-Filho , V. Pharmazie 2000 , 55 , 942 .
- Wanner , M. J. ; Koomen , G.-J. Tetrahedron 1991 , 47 , 8431 .
- Akula , M.R. ; Kabalka , G.W . Synth. Commun . 1998 , 28 , 2063 .
- Sondhi , S.M. ; Rani , R. Green Chem. Lett. Rev . 2010 , 3 , 115 ; Sondhi , S.M. ; Rani , R. Lett. Org. Chem . 2008 , 5 , 51 .
- Sondhi , S.M. ; Rani , R. ; Roy , P. ; Agrawal , S.K. ; Saxena , A.K. Bioorg. Med. Chem. Lett , 2010 , 20 , 2306 . Sondhi , S.M. ; Rani , R. ; Roy , P. ; Agrawal , S.K. ; Saxena , A.K. ; Eur. J. Med. Chem , 2010 , 45 , 902 .
- Vogel , A.I. 1968 . A Text book of Practical Organic Chemistry , 943 London : ELBS .
- Kolodynka , Z. ; Krupa , A. ; Wieniawski , W. Acta Poloniae Pharmaceut . 1979 , 36 , 256 .
- Verma , S.M. ; Sinha , K.O.P. Ind. J. Chem . 1974 , 12 , 586 .
- Shrivastava , A. ; Samtani , S. ; Verma , S.M. Proc. Ind. Natl. Sci. Acad . 1994 , 60A , 447 .
Electronic Supporting Information
An expeditious, highly efficient, catalyst and solvent-free synthesis of 9,10-dihydro-anthracene-9,10-α,β-succiniimide derivatives
Reshma Rani, Surbhi Arya, Praveen Kilaru and Sham M. Sondhi*
Department of Chemistry, Indian Institute of Technology-Roorkee, Roorkee 247667, Uttaranchal, India
Copies of1 H (500 MHz) and GC-MS Spectra: Pages: 2–25