Abstract
An efficient, chemoselective, convenient, and straightforward methodology has been developed for the protection of C=O group of aldehydes/ketones as C=N moiety of hydrazones catalyzed by ZrOCl2·8H2O (10 mol%) in acetonitrile and the same catalyst in methanol oxidatively cleaves C=N moiety of hydrazones to provide parent carbonyl compounds in high yields. The reactions have been performed in aerobic condition. The catalyst is inexpensive, readily available, easy to handle, insensitive to air and moisture, easily recoverable and can be reused and importantly less toxic.
Introduction
As far as the synthesis of complex target molecules is concerned, protection–deprotection is essential Citation1 and hence serves as a central theme in organic chemistry Citation2. Hydrazone is regarded as one of the important protective moieties for carbonyl compound in multi-step synthesis of many target molecules Citation3. Apart from carbonyl protection they also serve as carbanion equivalents in C–C bond forming reactions Citation4 Citation5. Accordingly, lots of efforts have been exercised for the development of a mild and efficient methodology for masking carbonyl moiety of aldehydes/ketones in the form of hydrazone and the subsequent oxidative cleavage of hydrazones to regenerate the parent carbonyl compounds. But, some of the reported methods Citation6–21 have one or the other limitations such as requirement of strongly oxidizing or reducing acidic or basic reagents, use of reagents in stoichiometric amounts or more, tedious work up procedure, low yield, longer reaction time, harsh reaction condition, lack of selectivity, associated with environmental hazard and importantly, tolerability to other functional groups. It is also likely that by product formed in the reaction may block the active sites of the catalyst thereby reducing its activity. Importantly, presence of sensitive structural features in molecules restricts the choice of reagents. Hence, there has been considerable interest in this area and still scope is there for further development.
The application of ZrOCl2·8H2O as a catalyst in organic synthesis has attracted our attention as it is relatively cheaper, readily available, easy to handle, insensitive to air and moisture Citation22 and importantly less toxic Citation23. This octahydrate of Zirconium oxychloride is a mild Lewis acid having some distinct differences Citation24 Citation25 from other metal hydrates. An account by Zhang et al. recently reviewed effectiveness of Zirconium-based compounds in many organic transformations, for example addition reaction, rearrangement reaction, protection of common functional groups such as carbonyl, carboxylic acid, amino and hydroxy groups Citation26 and their subsequent deprotection. Zolfigol et al. described the application of zirconium compounds in deprotection, oxidation, C–C, C–N, and C–O bond forming reactions Citation27. Catalytic activity of ZrOCl2·8H2O has been described for the oxidation of alcohols Citation28; acylation of alcohols, phenols, amines, and thiols Citation29; esterification of long chain carboxylic acids and alcohols Citation30; enaminones and enamino ester synthesis Citation31; chemoselective synthesis of 2-aryloxazolines and bis-oxazolines Citation32; and synthesis of benzoxazoles, benzothiazoles, benzimidazoles, and oxazolo[4,5-b]pyridines Citation33.
Results and discussion
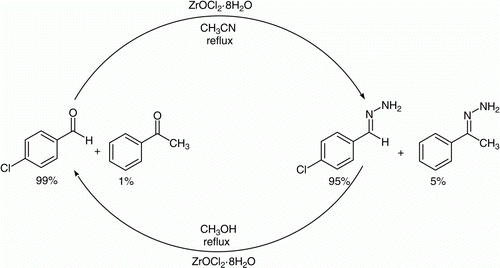
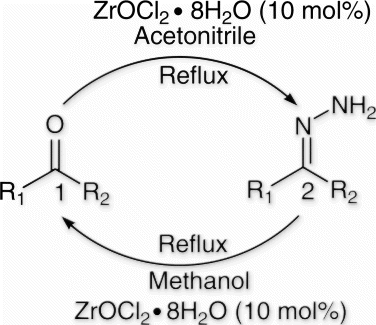
In continuation to our interest in protection and deprotection Citation34 and zirconium chemistry Citation35, we want to divulge herein a new and convenient protocol for the protection of aldehydes/ketones as corresponding hydrazones in refluxing acetonitrile and subsequent deprotection of hydrazones back to their parent carbonyl compounds in refluxing methanol using ZrOCl2·8H2O as catalyst (10 mol%) ().
In order to search for the optimum experimental condition, the reaction of benzaldehyde (1a) and hydrazine hydrate (1b) was chosen as the model reaction (). The need for a catalyst was realized by the observation that very poor yields (trace-19%) were obtained when the reactions were carried out in the absence of a catalyst at room temperature or heating at 80 °C under neat condition or refluxing in Dimethyl formamide/Dimethyl sulfoxide (DMF/DMSO) for 24 h. Various compounds were screened for their effectiveness as catalysts () in the model reaction. In addition, various solvents were also screened.
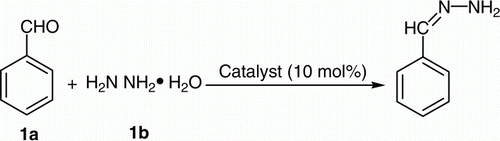
Table 1. Reaction of 1a and 1b in the presence of various catalysts.
The best result was obtained when we used 10 mol% of ZrOCl2·8H2O in refluxing acetonitrile for 2 h (, entry 17, yield 93%). In solvents such as Dichloromethane (DCM), DMSO, DMF and Tetrahydrofuran (THF), the reaction required longer time to afford either very poor yield (trace-15%) or no reaction at all.
In addition, to determine the effective catalyst loading, we employed varying amounts (1, 2, and 5 mol%, respectively) of ZrOCl2·8H2O in the model reaction, but the reaction did not undergo. However, using 7 mol% of ZrOCl2·8H2O under the same reaction condition, 52% yield was obtained. When the catalyst loading was increased to 10 mol%, to our delight the reaction underwent efficiently resulting excellent yield (, entry 5, 93%) of the product. Further increasing the catalyst to 15 and 20 mol% resulted lower yield (60 and 57%, respectively) requiring longer reaction times.
Table 2. Optimization of the catalyst ZrOCl2·8H2O for the protection of carbonyl functionality.
Encouraged by these results, next, the reaction of 1a with several hydrazine sources were carried out in the presence of ZrOCl2·8H2O (10 mol%) to investigate the influence of various hydrazine sources (). The reaction was found to be faster with hydrazine hydrate than with hydrazine sulfate and hydrazine chloride. Notably, with hydrazine nitrate no reaction occurred. The reaction of 1a with hydrazine hydrate in the presence of 10 mol% ZrOCl2·8H2O under neat condition provided low yield (, entry 7, 40%), whereas in case of hydrazine sulfate and hydrazine chloride the reaction was very slow providing only trace conversion to the product.
Table 3. Different hydrazine sources tested for the formation of hydrazones.
In an attempt to broaden the scope of the methodology and to establish the generality of the reaction, a range of aldehydes/ketones were reacted with hydrazine hydrate in the presence of 10 mol% ZrOCl2·8H2O in refluxing acetonitrile and the results are summarized in (entries 1–24). Entries 1–22 provided corresponding hydrazones in relatively good yields, except the entries 19 and 21. Groups such as −Cl,−NO2,−OH,−OMe, and −COOEt (, entries 2, 4, 6, 7, 10, 11, 15, and 19, respectively) were found to be compatibility with the reaction condition. For substituent at ortho-position, steric hindrance to the incoming nucleophile, probably played a role in lowering the yield (, entry 11). Again, substituent at meta-position deactivates the benzene ring which might be responsible for decreasing the yield (, entry 7).
Table 4. Protection of carbonyl compounds 1 by ZrOCl2·8H2O vide .
Among the aliphatic carbonyl compounds, those with shorter alkyl chains showed a higher yield (, entry 16) as compared to those with longer alkyl chain (, entries 17 and 18, respectively). Good chemoselectivity was observed for the substrate containing −COOEt group that did not experience any competition with the existing carbonyl group in the substrate (, entry 19), although yield was low (30%). In case of dicarbonyl compounds, when one equivalent of hydrazine hydrate was used, the reaction underwent producing corresponding mono-hydrazones in moderate yield (, entries 20 and 21, respectively). When two equivalents of hydrazine hydrate were used for masking both the carbonyl moieties, we did not observe any reaction occurring at both the carbonyl groups, that is selectively only mono-hydrazones were obtained. We believe that the active species, zirconium cation cluster [Zr4(OH)8(H2O)16]8 + is too bulky to co-ordinate with the two carbonyl groups at the same time. Hence, complexation with only one carbonyl group is possible and thus showing the chemoselectivity. The acyclic ketone, cyclohexanone (, entry 22) also provided good yield under the present reaction condition. Furthermore, the reaction was also found to proceed for the heteroatom containing aldehydes (, entries 12 and 13, respectively) with high yields. However, both benzil and benzoin (, entries 23 and 24, respectively) remained inert under the reaction condition. In general, aromatic compounds showed better yields than the aliphatic or acyclic ones.
In order to find the selectivity of the reaction, we investigated the competitive reaction: protection of 4-chlorobenzaldehyde in the presence of acetophenone (in equimolar amounts) using ZrOCl2·8H2O (10 mol%) in acetonitrile under refluxing condition. We have found that 4-chlorobenzaldehyde was converted to the corresponding hydrazone selectively and faster than acetophenone. Alternatively, in the competitive reaction using equimolar amounts of hydrazones of 4-nitrobenzaldehyde and acetophenone, under the same reaction condition, 4-nitrobenzaldehyde hydrazone was cleanly and selectively cleaved in the presence of acetophenone hydrazone ().
Alternatively, to extend our study, we also investigated oxidative deprotection of hydrazones 2 to yield the corresponding carbonyl compounds 1 catalyzed by ZrOCl2·8H2O (10 mol%) in refluxing methanol (). The cleavage of hydrazones was equally effective for both aldehydes and ketones as summarized in (entries 1–22). Interestingly, deprotection of hydrazones was much faster than the protection of carbonyl compounds. Both hydroxyl and nitro groups present in the compounds did not have any observable effect on the yields of the products (, entries 6, 7, 10 and 11, respectively). The deprotection was also found to proceed efficiently for the hetero atom containing hydrazones (, entries 12 and 13, respectively). It was also observed that the substrate having either electron donating or electron withdrawing groups on the aromatic ring reacted with varying reaction times (, entries 2, 4, and 6–11, respectively). The hydrazones of aliphatic aldehydes were also cleanly cleaved by ZrOCl2·8H2O under the present reaction condition (, entries 16–18, respectively). The deprotection of hydrazone of acyclic ketone took place with good yield (, entry 22). However, regeneration of corresponding carbonyl compounds from the hydrazones of α,β-unsaturated aldehyde, p-alkoxybenzaldehyde and alkylacetoacetate (, entries 14, 15, and 19, respectively) failed under the reaction condition. The actual mechanism of the reaction is not clear to us at this stage. Notably the aldehydes/ketones regenerated from their hydrazones did not undergo further oxidation under the present reaction condition.
Table 5. Deprotection of hydrazones 2 by ZrOCl2·8H2O.
A range of protic solvents was screened in order to evaluate the scope and limitation as far as the deprotection of hydrazones into their parent carbonyl compounds using ZrOCl2·8H2O (10 mol%) () is concerned. For this purpose, 4-chlorobenzaldehyde hydrazone was chosen as the model reactant with 10 mol% ZrOCl2·8H2O in various solvents. Methanol was found to be the choice of solvent among all the solvents with faster conversion and quantitative yield.
Table 6. Effect of solvents for ZrOCl2·8H2O catalyzed deprotection of hydrazones.
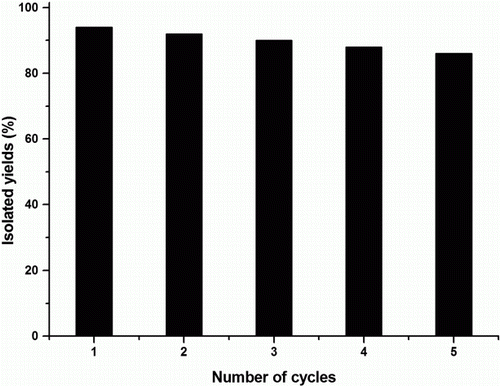
It is also noteworthy to mention that the catalyst is recyclable, an important aspect from green chemistry and industrial point of view. After the aqueous work-up, ZrOCl2·8H2O was recovered from the aqueous solution by concentrating under reduced pressure. Alternatively, after filtration, the catalyst was washed with CH2Cl2 or CHCl3, dried at 60 °C and subjected to reaction again. Even after five runs for protection and deprotection reaction, the catalytic activity of ZrOCl2·8H2O was found to be almost same as that of freshly used catalyst (), without significant loss of its activity. The catalyst recyclability chart is shown below ().
ZrOCl2·8H2O is regarded to be an ionic cluster of [Zr4(OH)8(H2O)16]Cl8·12H2O; and the zirconium cation cluster [Zr4(OH)8(H2O)16]8 + is usually thought to be active species Citation36 Citation37. The comparison of the X-ray diffraction (XRD) patterns of the fresh catalyst with the standard one reveals that the catalyst, which we have used is actually a mixture of ZrOCl2·8H2O and ZrOCl2·6H2O. In the Fourier Transform Infra-Red (FT-IR) spectrum, the peak at 1621 cm−1 is a characteristic peak for the ZrOCl2·8H2O as per literature Citation38. Broad absorption peak was observed in the range 3400–3520 cm−1 both in the catalyst and in the recovered catalyst. However, for the recovered catalyst, a small new peak at 1029.91 cm−1 is observed Citation38.
In conclusion, we have developed a simple methodology for the protection of aldehydes/ketones into hydrazones and deprotection of hydrazones back to corresponding aldehydes/ketones under non-aqueous and protic condition catalyzed by ZrOCl2·8H2O (10 mol%). Moreover, this is a good development as far as chemistry of Zirconium is concerned.
Experimental
General
1H-NMR and 13C-NMR spectra were recorded on a JNM ECS 400 MHz (JEOL) spectrophotometer in CDCl3 with Tetramethylsilane (TMS) as the internal standard. IR spectra were recorded on a Nicolet (Impact 410) FT-IR spectrophotometer using KBr disks. Mass spectra were recorded on a Waters Q-TOF Premier & Aquity UPLC spectrometer. Melting points/boiling points were checked on a Büchi 504 apparatus and are uncorrected. All the chemicals were used as received. ZrOCl2·8H2O was purchased from Fluka. All reactions were monitored by TLC on silica gel 60 F254 (0.25 mm), visualization was effected with UV and/or by developing in iodine. Purification of the reaction products was carried out by column chromatography using 60–120 mesh silica gel.
Typical procedure for the protection of carbonyl compounds 1 into hydrazones 2 using ZrOCl2·8H2O vide
In an oven-dried round-bottomed flask (50 mL), benzaldehyde (1 mmol), hydrazine hydrate (1 mmol), and ZrOCl2·8H2O (10 mol%) were taken and acetonitrile (5 mL) was added to it. The reaction mixture was refluxed for required time under aerobic condition. After the completion of the reaction (monitored by TLC), the reaction system was allowed to cool to room temperature, acetonitrile was removed in a rotary evaporator and then extracted with ethyl acetate (3×10 mL), washed with water and brine, dried over Na2SO4, concentrated in a rotary evaporator and subjected to column chromatography to furnish the pure product (Yield 93%). Finally, the product was recrystalized from ethanol to afford the purest product, which was identified by IR, 1H, 13C-NMR, mass, mp, and compared with authentic sample.
Typical procedure for the deprotection of hydrazones 2 into their parent aldehydes/ketones 1using ZrOCl2·8H2O vide
The benzaldehyde hydrazone (1 mmol) and ZrOCl2·8H2O (10 mol%) were taken in an oven-dried round-bottomed flask (50 mL) and methanol (5 mL) was added to it. It was then refluxed for the appropriate time under aerobic condition. After the completion of the reaction (vide TLC), the reaction mixture was allowed to cool to room temperature, methanol was removed in a rotary evaporator and then extracted with ethyl acetate (3×10 mL), washed with water and brine, dried over Na2SO4, concentrated in a rotary evaporator and finally performed column chromatography to afford the pure product (Yield 95%). The product was identified by IR, 1H, 13C-NMR, mass, bp and compared with authentic samples.
When the product was solid, recrystalization from ethanol provided the pure compound.
Acknowledgements
AJT thanks Council of Scientific and Industrial Research, New Delhi, India for financial support. VKD and SD thank University Grants Commission, New Delhi, India for Rajiv Gandhi fellowship.
References
- Jarowicki , K. ; Kocienski , P. J. Chem. Soc., Perkin Trans . 1998 , 1 , 4005 .
- Greene , T.W. ; Wuts , P.G.M. Protective Groups in Organic Synthesis , 3rd ed , Wiley , New York , 1999 , p 49 .
- Bergeiter , D.E. ; Momongam , M. Comprehensive Organic Synthesis ; Pergamon Press , Oxford , 1991 , Vol. 2 , p 503 .
- Cuvigny , T. ; Normant , H. Synthesis 1977 , 3 , 198
- Enders , D. ; Eichenauer , H. Angew. Chem. Int. Ed. Engl . 1997 , 15 , 549 .
- Ballini , R. ; Petrini , M. J. Chem. Soc., Perkin. Trans . 1988 , 1 , 2563 .
- Hajipour , A.R. ; Mallakpour , S.E. ; Beltork , I.M. ; Adibi , H. Synth. Commun . 2001 , 31 , 3401 .
- Yadav , J.S. ; Reddy , S.V.B. ; Reddy , K.S.M. ; Sabitha , G. Synlett 2001 , 7 , 1134 .
- Kumar , P. ; Hegde , R.V. ; Pandey , B. ; Ravindranathan , T. J. Chem. Soc., Chem. Commun . 1993 , 20 , 1553 .
- Adlington , M.R. ; Baldwin , J.E. ; Bottaro , C.J. ; Perry , D.W.M. J. Chem. Soc., Chem. Commun . 1983 , 18 , 1040 .
- Mino , T. ; Hirota , T. ; Yamashita , M. Synlett 1996 , 10 , 999 .
- Laszlo , P. ; Polla , E. Tetrahedron Lett . 1984 , 25 , 3309 .
- Olah , G.A. ; Vankar , D.Y. ; Prakash , G.K.S. Synthesis 1979 , 2 , 113 .
- Jung , C.J. ; Kim , S.K. ; Kim , Y.H. Synth. Commun . 1992 , 22 , 1583 .
- Kamal , A. ; Rao , V.M. ; Meshram , M.H. Tetrahedron Lett . 1991 , 32 , 2657 .
- Barhate , B.N. ; Gajare , A.S. ; Wakharkar , D.R. ; Sudalai , A. Tetrahedron Lett . 1997 , 38 , 653 .
- Shirini , F. ; Mamaghani , M. ; Rahmanzadeh , A. Arkivoc 2007 , i , 34 .
- Petroski , J.R. Synth. Commun . 2006 , 36 , 1727 .
- Mino , T. ; Hirota , T. ; Fujita , N. ; Yamashita , M. Synthesis 1999 , 12 , 2024 .
- Enders , D. ; Wortmann , L. ; Peters , R. Acc. Chem. Res . 2000 , 33 , 157 .
- Heravi , M.M. ; Sabaghian , J.A. ; Bakhtiari , K. ; Ghassemzadeh , M. J. Braz. Chem. Soc . 2006 , 17 , 1 .
- Farnworth , F. ; Jones , S.L. ; McAlpine , I. Speciality Inorganic Chemicals ; Special Publication No. 40 , Royal Society of Chemistry , London , 1980 .
- Lewis , R.J.S.R. ., Dangerous Properties of Industrial Materials , 8th ed , Van Nostrand Reinhold , New York , 1989 , 3 .
- Rambo , C.R. ; Cao , J. ; Sieber , H. Mater. Chem. Phys . 2004 , 87 , 345 .
- Dondi , M. ; Fabbri , B. ; Mingazzini , C. Talanta 1998 , 45 , 1201 .
- Zhang , Z.-H. ; Li , T.-S. Curr. Org. Chem . 2009 , 13 , 1 .
- Mohammad , A.Z. ; Farhad , S. ; Peyman , S. ; Masoumeh , A. Curr. Org. Chem . 2008 , 12 , 183 .
- Shirini , F. ; Zolfigol , E. ; Mollarazi , E. Synth. Commun . 2005 , 35 , 1541 .
- Ghosh , R. ; Maiti , S. ; Chakraborty , A. Tetrahedron Lett . 2005 , 46 , 147 .
- Mantri , K. ; Komura , K. ; Sugi , Y. Green Chem . 2005 , 7 , 677 .
- Zhang , Z.-H. ; Li , T.-S. ; Li , J.-J. Catal. Commun . 2007 , 8 , 1615 .
- Baltork , M.I. ; Khosropour , R.A. ; Hojati , F.S. Catal. Commun . 2007 , 8 , 200 .
- Baltork , M.I. ; Khosropour , R.A. ; Hojati , F.S. Catal. Commun . 2007 , 8 , 1865 .
- Das , S. ; Bora , R. ; Devi , R.R. ; Thakur , A.J. Synlett 2008 , 18 , 2741 .
- Talukdar , D. ; Saikia , L. ; Thakur , A.J. Synlett 2011 , 11 , 1597 .
- Nakayama , M. ; Sato , A. ; Ishihara , K. ; Yamamoto , H. Adv. Synth. Catal . 2004 , 346 , 1275 ; Sun, H.B.; Hua, R.M.; Yin, Y.W. Molecules 2006, 11, 263; Firouzabadi, H.; Iranpoor, N.; Jafarpour, M.; Ghaderi, A. J. Mol. Catal. A Chem. 2006, 252, 150; Rodriguez-Dominguez, J.C.; Bernardi, D.; Kirsch, G. Tetrahedron Lett. 2007, 48, 5777 .
- Bhagat , S. ; Chakraborti , A.K. J. Org. Chem . 2008 , 73 , 6029 .
- Wu , Y. ; He , L.N. ; Du , Y. ; Wang , J.Q. ; Miao , C.X. ; Li , W. Tetrahedron 2009 , 65 , 6204 .