Abstract
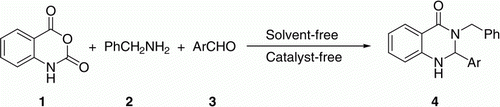
An efficient and convenient method for the preparation of 3-benzylquinazolin-4(1H)-one derivatives under solvent-free and catalyst-free conditions by the reaction of isatoic anhydride, benzylamine, and aromatic aldehydes was reported. In reported papers, ammonia water, ammonium salt, and aromatic amine were often used to synthesize quinazolin-4(1H)-one derivative, but benzylamine was seldom used in this synthesis. This article offers a green method for the synthesis of 3-benzylquinazolin-4(1H)-one derivative used benzylamine as starting material. This methodology has the advantages of short reaction time, mild reaction conditions, easy work-up, and environmental friendliness.
Introduction
It is well known that heterocycles are abundant in nature and are of great significance to life because their structural subunits exists in many natural products such as vitamins, hormones, antibiotics, as well as pharmaceuticals, herbicides, dyes, and many other compounds Citation1.
Quinazolin-4(1H)-one derivatives, as the important heterocyclic compounds, have drawn much attention due to their various biological and medicinal activities, and they can be used as anticonvulsant, antibacterial, antidiabetic, anticarcinogen, and other biological or medicinal agents Citation2 Citation3.
There are several methods for the preparation of this class of compounds. The typical procedure for the synthesis of quinazolin-4(1H)-one involves 2-aminobenzoic or 2-nitrobenzoic acid derivatives or 2-aminobenzonitrile derivatives or 2-halophenyl precursors and others reagents. The availability of these special starting materials constrains the application of these methods as follows: In 2008, Su Citation4 reported the synthesis of 2,3-dihydroquinazolin-4(1H)-ones and quinazolin-4(3H)-ones by one-pot synthesis catalyzed by Ga(OTf)3 in ethanol. Salehi and his co-workers reported the synthesis of such compounds involving different catalysts, such as: p-toluenesulfonic acids Citation5, silica sulfuric acid Citation6, alum Citation7, and Montmorillonite K-10 Citation8. Synthesis of new 2-aryl substituted 2,3-dihydroquinazolin-4(1H)-ones were reported by Rostamizadeh under solvent-free conditions, using molecular iodine as catalyst at high temperature Citation9. Several years ago, Shi reported one-pot synthesis of quinazolin derivatives using TiCl4-Zn as catalyst in anhydrous THF Citation10. Some other methods for the synthesis of quinazolin derivatives involved the condensation of 2-aminobenzamide with aldehydes or ketones in the presence of multiple catalysts such as CuCl2 Citation11, NH4Cl Citation12, Sc(OTf)3 Citation13, SmI2 Citation14, Zn(PFO)2 Citation15 and so on. Most of the reported methods often suffer from tedious procedures and low yields. For example, if the reaction was promoted by TiCl4-Zn Citation10, it must be operated under oxygen-free and anhydrous conditions. Even little amount of oxygen could stop the reaction from happening. When it comes to Zn(PFO)2 Citation15, the reaction time was very long and the reaction should be performed in H2O/EtOH under refluxing. Furthermore, some catalysts are not easily available and they are very costly. However, the most disadvantages about the reported methods were that the organic solvent was required, and this would contaminate our living surroundings. Therefore, simpler and high yield approach toward this valuable nucleus is much desirable. In addition, in reported papers, ammonia water Citation5, 16–18, ammonium salt (5–9, 19), and aromatic amine Citation6, 15 were often used to synthesize quinazolin-4(1H)-one derivative, but benzylamine was seldom used in this synthesis. Only one paper Citation20 has been reported about benzylamine to synthesis of quinazolin-4(1H)-one so far.
Facing with the ever-growing concern for environmental issues, methods with traditional organic synthesis has been greatly challenged. Thus, it places higher expectations on the future work for all the organic chemists. In this context, organic reactions under solvent-free conditions are ideal protocols for the development of environmental friendly and economical advantageous chemical processes. Such methods can not only meet the requirements of sustainable development, but also reflects the concept of low-carbon economy. There has been an upsurge of interest in synthesizing compounds in a solvent-free environment during recent years Citation21–23. Compared with the ways employed in the solvent, the solvent-free approach proceeded more cleanly and provided higher yields. Multicomponent reactions (MCRs) Citation24–28 are special types of synthetically useful organic reactions in which three or more different starting materials react to a final product in a one-pot procedure. If the one-pot, MCRs could be carried out under solvent-free conditions and that must be an ideal synthetic method. In continuation of our ongoing endeavor to synthesize heterocyclic compounds by MCRs under solvent-free conditions Citation29–31, herein, we report a three-component one-step synthesis of 3-benzylquinazolin-4(1H)-one derivatives under catalyst- and solvent-free conditions.
Results and discussion
The process could be depicted as follows: isatoic anhydride 1, benzylamine 2, aromatic, and aldehyde 3 were mixed and ground in a mortar (). The mixture was heated at 70°C under solvent-free and catalyst-free conditions for about 10 min and the reactions could be completed, and the corresponding 3-benzyl-2-aryl-2,3-dihydroquinazolin-4(1H)-one derivatives were obtained in excellent yields. The progress of the reaction was monitored by thin-layer chromatography (TLC).
Then we explored the scope of our reaction conditions by applying the optimal reaction conditions to a number of aromatic aldehydes bearing electron-withdrawing and electron-donating substituents and found that the property of substituent groups of the aromatic aldehydes did not affect these reactions. The results of the reaction were listed in .
Table 1. The results of synthesis of 3-benzylquinazolin-4(1H)-one derivatives.
It is worth mentioning that this reaction was carried out under catalyst-free conditions. Benzylamine, as one of the reactants, may have the role of catalyst, therefore, the reaction do not need other catalysts. This is the ideal method for organic synthesis, because in reported literatures Citation5–20 the catalysts were requisite and they would increase the difficulty of purification products, however, this operation could be omitted in our process. From the 1H NMR spectra, we could find that hydrogen of benzyl has taken place geminal coupling, the chemical shifts of two hydrogen atoms are about 3.70 ppm and 5.30 ppm, respectively and the coupling constants are about J=15.2 Hz and J=15.6 Hz. This property accords with the structure of product.
The structures of all the products were confirmed on the basis of spectroscopic data, particularly 1H NMR analysis and HRMS spectra. Some new reported compounds have been afforded 13C NMR datum.
In summary, in this paper, we have described a simple one-pot three component reaction involving isatoic anhydride 1, benzylamine 2 aromatic aldehydes 3 under solvent-free conditions for the synthesis of 3-benzylquinazolin-4(1H)-one derivatives. Particularly, valuable features of this method include the mild reaction conditions, low cost, operational simplicity and reduced environmental impact. Moreover, the products were performed in excellent yields within short reaction times and the reaction could be carried out without any catalyst. Starting materials are also inexpensive and commercially available.
Experimental
Melting points were determined on XT-5 microscopic melting-point apparatus and were uncorrected. IR spectra were recorded on a FT IR-8101 spectrometer. 1H NMR spectra were obtained from solution in DMSO-d 6 with Me4Si as internal standard using a Bruker-400 spectrometer. HRMS spectra were obtained with a Bruker microOTOF-Q 134 instrument.
General procedure for the synthesis of 3-benzyl-2-aryl-2,3-dihydroquinazolin-4(1H)-one derivatives
To a 100 mL dried round bottom flask were added isatoic anhydride 1 (2 mmol), benzylamine 2 (3 mmol) and aromatic aldehydes 3 (2 mmol). The mixture was heated at approx. 70°C about 8–15 min (the reaction was monitored by TLC). After the reaction was completed, the reaction mixture was poured into water, and then washed with water thoroughly. The product was filtered, dried, and recrystallized from 95% ethanol.
Spectral data of compounds
3-Benzyl-2-(4-fluorophenyl)-2,3-dihydroquinazolin-4(1H)-one (4a): m.p. 117~118°C; IR (KBr, ν, cm−1): 3358, 1632, 1576, 1554, 1448, 1418, 1362, 1324, 1269, 1150, 753, 727 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.83 (1H, d, J=15.2 Hz, PhCH), 5.22 (1H, d, J=15.2 Hz, PhCH), 6.04 (1H, s, ArCH), 6.69 (1H, d, J=8.0 Hz, ArH), 6.74 (1H, t, J=8.0 Hz, ArH), 7.23–7.28 (5H, m, ArH), 7.30–7.34 (4H, m, ArH), 7.38 (1H, dd, J=2.0 Hz, J=8.4 Hz, ArH), 7.66 (1H, d, J=2.0 Hz, ArH), 7.76 (1H, dd, J=1.2 Hz, J=8.0 Hz, NH); HRMS m/z calculated for C21H17FN2O [M + H]: 333.1403, found: 333.1403.
3-Benzyl-2-(4-bromophenyl)-2,3-dihydroquinazolin-4(1H)-one (4b): m.p. 135~137°C; IR (KBr, ν, cm−1): 3302, 1644, 1557, 1539, 1506, 1488, 1455, 1409, 1359, 1292, 1161, 830, 755, 695 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.88 (1H, d, J=15.2 Hz, PhCH), 5.30 (1H, d, J=15.6 Hz, PhCH), 5.77 (1H, s, ArCH), 6.65 (1H, d, J=8.0 Hz, ArH), 6.70 (1H, t, J=8.0 Hz, ArH), 7.21–7.36 (8H, m, ArH), 7.43 (1H, s, ArH), 7.55 (2H, d, J=8.0 Hz, ArH), 7.70 (1H, dd, J=1.6 Hz, J=8.0 Hz, NH); HRMS m/z calculated for C21H17BrN2O [M + H]: 393.0603, found: 393.0595.
3-Benzyl-2-(3-chlorophenyl)-2,3-dihydroquinazolin-4(1H)-one (4c): m.p. 146~148°C; IR (KBr, ν, cm−1): 3303, 1635, 1558, 1541, 1496, 1450, 1419, 1355, 1317, 1289, 1238, 1190, 1154, 833, 755, 683 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.94 (1H, d, J=15.6 Hz, PhCH), 5.30 (1H, d, J=15.2 Hz, PhCH), 5.83 (1H, s, ArCH), 6.67 (1H, d, J=8.4 Hz, ArH), 6.71 (1H, d, J=8.4 Hz, ArH), 7.22–7.27 (3H, m, ArH), 7.29–7.34 (4H, m, ArH), 7.35–7.37 (3H, m, ArH), 7.47 (1H, s, ArH), 7.71 (1H, dd, J=1.6 Hz, J=8.0 Hz, NH); 13C NMR (400 MHz, CDCl3) 46.95, 70.24, 114.45, 115.35, 119.24, 124.50, 126.69, 127.51, 127.83, 128.61, 128.64, 129.28, 130.17, 133.76, 134.75, 136.36, 141.43, 144.80, 163.07; HRMS m/z calculated for C21H17ClN2O [M + H]: 349.1108, found: 349.1109.
3-Benzyl-2-(4-chlorophenyl)-2,3-dihydroquinazolin-4(1H)-one (4d): m.p. 125~126°C; IR (KBr, ν, cm−1): 3298, 1633, 1489, 1411, 1164, 1091, 1014, 948, 830, 754, 726, 695 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.88 (1H, d, J=15.6 Hz, PhCH), 5.29 (1H, d, J=15.2 Hz, PhCH), 5.78 (1H, d, J=2.4 Hz, ArCH), 6.65 (1H, d, J=8.0 Hz, ArH), 6.70 (1H, t, J=8.0 Hz, ArH), 7.24–7.28 (3H, m, ArH), 7.30–7.33 (5H, m, ArH), 7.40–7.42 (3H, m, ArH), 7.70 (1H, d, J=8.0 Hz, NH); HRMS m/z calculated for C21H17ClN2O [M + Na]: 371.0927, found: 371.0939.
3-Benzyl-2-(2,4-dichlorophenyl)-2,3-dihydroquinazolin-4(1H)-one (4e): m.p. 142~144°C; IR (KBr, ν, cm−1): 3301, 1627, 1584, 1558, 1522, 1456, 1291, 1241, 1162, 1103, 756, 698 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.83 (1H, d, J=15.2 Hz, PhCH), 5.22 (1H, d, J=15.2 Hz, PhCH), 6.04 (1H, s, ArCH), 6.69 (1H, d, J=8.0 Hz, ArH), 6.74 (1H, t, J=8.0 Hz, ArH), 7.23–7.29 (5H, m, ArH), 7.31–7.33 (3H, m, ArH), 7.38 (1H, dd, J=2.0 Hz, J=8.4 Hz, ArH), 7.66 (1H, d, J=2.0 Hz, ArH), 7.76 (1H, dd, J=1.2 Hz, J=8.0 Hz, NH); 13C NMR (400 MHz, CDCl3) 47.54, 66.74, 114.58, 115.42, 119.45, 126.80, 127.44, 127.97, 128.26, 128.63, 128.68, 128.83, 129.96, 130.50, 132.32, 133.80, 134.60, 136.13, 138.42, 144.12, 163.37; HRMS m/z calculated for C21H16Cl2N2O [M + H]: 383.0718, found: 383.0711.
3-Benzyl-2-(3,4-dichlorophenyl)-2,3-dihydroquinazolin-4(1H)-one (4f): m.p. 115~116°C; IR (KBr, ν, cm−1): 3297, 1626, 1515, 1489, 1417, 1304, 1029, 834, 755, 685 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 4.01 (1H, d, J=15.2 Hz, PhCH), 5.24 (1H, d, J=15.2 Hz, PhCH), 5.86 (1H, s, ArCH), 6.67 (1H, d, J=8.4 Hz, ArH), 6.72 (1H, t, J=8.0 Hz, ArH), 7.23–7.33 (7H, m, ArH), 7.47 (1H, s, NH), 7.54 (1H, d, J=1.6 Hz, ArH), 7.60 (1H, d, J=8.4 Hz, ArH), 7.71 (1H, d, J=7.2 Hz, ArH); 13C NMR (400 MHz, CDCl3) 47.10, 69.72, 114.60, 115.36, 119.45, 125.62, 127.74, 127.77, 128.53, 128.55, 128.62, 128.66, 130.80, 132.98, 133.18, 133.89, 136.16, 139.61, 144.62, 163.03; HRMS m/z calculated for C21H16Cl2N2O [M + H]: 383.0718, found: 383.0702.
3-Benzyl-2-p-tolyl-2,3-dihydroquinazolin-4(1H)-one (4g): m.p. 129~130°C; IR (KBr, ν, cm−1): 3259, 1624, 1520, 1495, 1430, 1327, 1291, 1241, 1161, 748, 699 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 2.26 (3H, s, CH3), 3.79 (1H, d, J=15.2 Hz, PhCH), 5.35 (1H, d, J=15.2 Hz, PhCH), 5.70 (1H, s, ArCH), 6.65 (1H, J=8.4 Hz, ArH), 6.69 (1H, t, J=8.4 Hz, ArH), 7.15 (2H, J=8.0 Hz, ArH), 7.21 (3H, J=8.0 Hz, ArH), 7.27–7.36 (6H, m, ArH), 7.71 (1H, dd, J=1.6 Hz, J=8.0 Hz, NH); HRMS m/z calculated for C22H20N2O [M + H]: 329.1654, found: 329.1693.
3-Benzyl-2-(2-methoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one (4h): m.p. 137~139°C; IR (KBr, ν, cm−1): 3261, 1629, 1516, 1488, 1460, 1238, 1151, 1111, 1077, 1027, 947, 827, 694 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.68 (1H, d, J=15.2 Hz, PhCH), 3.79 (3H, s, OCH3), 5.36 (1H, d, J=15.6 Hz, PhCH), 5.96 (1H, s, ArCH), 6.66 (1H, d, J=8.0 Hz, ArH), 6.70 (1H, t, J=8.0 Hz, ArH), 6.87 (1H, t, J=8.0 Hz, ArH), 6.94 (1H, s, ArH), 7.05–7.10 (2H, m, ArH), 7.18–7.36 (7H, m, ArH), 7.73 (1H, dd, J=1.6 Hz, J=8.0 Hz, NH); 13C NMR (400 MHz, CDCl3) 47.44, 55.35, 65.73, 65.78, 110.64, 114.53, 115.49, 118.70, 118.72, 120.62, 126.40, 126.53, 127.35, 127.65, 128.50, 128.56, 129.75, 133.37, 137.01, 145.65, 156.52, 163.95; HRMS m/z calculated for C22H20N2O2 [M + H]: 345.1603, found: 345.1599.
3-Benzyl-2-(4-methoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one (4i): m.p. 165~166°C; IR (KBr, ν, cm−1): 3295, 1633, 1503, 1485, 1420, 1404, 1352, 1309, 1255, 1176, 1030, 985, 933, 831, 759, 723, 694 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.72 (3H, s, OCH3), 3.80 (1H, d, J=15.2 Hz, PhCH), 5.30 (1H, d, J=15.6 Hz, PhCH), 5.69 (1H, s, ArCH), 6.64 (1H, J=8.0 Hz, ArH), 6.69 (1H, t, J=8.0 Hz, ArH), 6.90 (2H, d, J=8.8 Hz, ArH), 7.24 (3H, J=8.8 Hz, ArH), 7.27 (3H, J=8.0 Hz, ArH), 7.32–7.36 (3H, m, ArH), 7.70 (1H, dd, J=1.6 Hz, J=8.0 Hz, NH); HRMS m/z calculated for C22H20N2O2 [M + Na]: 367.1422, found: 367.1419.
3-Benzyl-2-(3,4,5-trimethoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one (4j): m.p. 177~179°C; IR (KBr, ν, cm−1): 3270, 1628, 1595, 1426, 1399, 1362, 1326, 1156, 1128, 1005, 836, 731, 693 cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.62 (3H, s, OCH3), 3.64 (6H, s, 2×OCH3), 4.07 (1H, d, J=15.6 Hz, PhCH), 5.08 (1H, d, J=15.6 Hz, PhCH), 5.73 (1H, s, ArCH), 6.61 (2H, s, ArH), 6.69 (1H, J = 8.0 Hz, ArH), 6.73 (1H, d, J=8.4 Hz, ArH), 7.22–7.33 (7H, m, ArH), 7.72 (1H, dd, J=1.6 Hz, J=8.0 Hz, NH); 13C NMR (400 MHz, CDCl3) 46.76, 55.74, 60.66, 72.04, 103.99, 114.25, 115.20, 118.76, 127.09, 127.61, 128.31, 128.46, 133.58, 134.26, 137.17, 138.11, 146.01, 152.99, 163.69; HRMS m/z calculated for C24H24N2O4 [M + H]: 405.1814, found: 405.1801.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (NSFC) (21172188), Foundation of Xuzhou Normal University (10XLS02) and the Priority Academic Program Development of Jiangsu Higher Education Institutions for financial support.
References
- Dou , G.L. ; Shi , D.Q. ; Li , Y.H. J. Comb. Chem . 2010 , 12 , 195 – 199 .
- Witt , A. ; Bergman , J. Curr. Org. Lett . 2003 , 7 , 659 – 677 .
- (a) Michael , J.P. Nat. Prod. Rep . 2008 , 25 , 166 – 187 . (b) Michael, J.P. Nat. Prod. Rep. 2007, 24, 223–246 .
- Chen , J.X. ; Wu , D.Z. ; He , F. ; Liu , M.C. ; Wu , H.Y. ; Ding , J.C. ; Su , W.K. Tetrahedron Lett . 2008 , 49 , 3814 – 3818 .
- Baghbanzadeh , M. ; Salehi , P. ; Dabiri , M. ; Kozehgarya , G. Synthesis . 2006 , 2 , 344 – 348 .
- Salehi , P. ; Dabiri , M. ; Zolfigol , M.A. ; Baghbanzadeh , M. Synlett . 2005 , 7 , 1155 – 1157 .
- Dabiri , M. ; Salehi , P. ; Otokesh , S. ; Baghbanzadeh , M. ; Kozehgarya , G. ; Mohammadi , A. Tetrahedron Lett . 2005 , 46 , 6123 – 6126 .
- Salehi , P. ; Dabiri , M. ; Baghbanzadeh , M. ; Bahramnejad , M. Synth. Commun . 2006 , 36 , 2287 – 2292 .
- Rostamizadeh , S. ; Amani , A.M. ; Aryan , R. ; Ghaieni , H.R. ; Shadjou , N. Synth. Commun . 2008 , 38 , 3567 – 3576 .
- Shi , D.Q. ; Rong , L.C. ; Wang , J.X. ; Zhuang , Q.Y. ; Wang , X.S. ; Hu , H.W. Tetrahedron Lett . 2003 , 44 , 3199 – 3201 .
- Abdel-Jalil , R.J. ; Voelter , W. ; Saeed , M. Tetrahedron Lett . 2004 , 45 , 3475 – 3476 .
- Shaabani , A. ; Maleki , A. ; Mofakham , H. Synth. Commun . 2008 , 38 , 3751 – 3759 .
- Chen , J.X. ; Wu , H.Y. ; Su , W.K. Chin. Chem. Lett . 2007 , 18 , 537 – 538 .
- Su , W.K. ; Yang , B.B. Aust. J. Chem . 2002 , 55 , 695 – 697 .
- Wang , L.M. ; Hu , L. ; Shao , J.H. ; Yu , J.J. ; Zhang , L. J. Fluorine Chem . 2008 , 129 , 1139 – 1145 .
- Minoo , D. ; Peyman , S. ; Mostafa , B. ; Mohammad , A.Z. ; Maria , A. ; Sedigheh , H. Cata. Commun . 2008 , 9 , 785 – 788 .
- Surpur , M.P. ; Singh , P.R. ; Patil , S.B. ; Samant , S.D. Synth. Commun . 2007 , 37 , 1965 – 1970 .
- Zhang , Z.H. ; Lu , H.Y. ; Yang , S.H. ; Gao , J.W. J. Comb. Chem . 2010 , 12 , 643 – 646 .
- Majid , G. ; Kobra , A. ; Hamed , M.P. ; Hamid Reza , S. Chin. J. Chem . 2011 , 29 , 1617 – 1623 .
- Karimi-Jaberi , Z. ; Arjmandi , R. Monatah. Chem . 2011 , 142 , 631 – 635 .
- Nguyen , T.B. ; Martel , A. ; Dhal , R. ; Dujardin , G. J. Org. Chem . 2008 , 73 , 2621 – 2632 .
- Yan , B. ; Lin , Y.H. Org. Lett . 2007 , 9 21 , 4323 – 4326 .
- Lee , H.G. ; Won , J.E. ; Kim , M.J. ; Park , S.E. ; Jung , K.J. ; Kim , B.R. ; Lee , S.G. ; Yoon , Y.J. J. Org. Chem . 2009 , 24 , 5675 – 5678 .
- Li , C.J. Chem. Rev . 1993 , 93 , 2023 – 2035 .
- Metzger , J. Angew. Chem. Int. Ed . 1998 , 37 21 , 2975 – 2978 .
- Tanaka , K. ; Toda , F. Chem. Rev . 2000 , 100 , 1025 – 1074 .
- Li , C.J. Chem.Rev . 2005 , 105 , 3095 – 3165 .
- Hobbs , H.R. Thomas , N.R. Chem. Rev . 2007 , 107 , 2786 – 2780 .
- Rong , L.C. ; Li , X.Y. ; Wang , H.Y. ; Shi , D.Q. ; Tu , S.J. Chem. Lett . 2006 , 35 , 1314 – 1315 .
- Rong , L.C. ; Han , H.X. ; Jiang , H. ; Tu , S.J. J. Heterocyclic Chem . 2009 , 46 , 465 – 468 .
- Rong , L.C. ; Gao , L.J. ; Han , H.X. ; Jiang , H. ; Dai , Y.S. ; Tu , S.J. Synth. Commun . 2010 , 40 , 289 – 294 .