Abstract
A facile and convenient procedure for the synthesis of 4H-pyrans from aldehydes, malononitrile, and ethyl acetoacetate/acetylacetone and also synthesis of 4H-pyrano[2,3-c]pyrazoles from three-component condensation of aldehydes, malononitrile, and pyrazolone or four-component condensation of aldehydes, malononitrile, ethyl acetoacetate, and hydrazine monohydrate using [bmim]OH as task-specific ionic liquid has been described. The protocol proves to be efficient and environmentally benign in terms of high yields, low reaction times, ease of recovery, and reusability of reaction medium.
Introduction
Multicomponent processes have recently gained considerable economic and ecological impetus as they address fundamental principles of synthetic efficiency and reaction design. Multicomponent reactions (MCRs) have been proven to be very elegant and rapid way to access complex structures in a single synthetic operation from simple building blocks and show high atom economy and high selectivity Citation1–6. The diversity, efficiency, and rapid access to small and highly functionalized organic molecules make this approach of central interest in the construction of combinatorial libraries and optimization of drug-discovery processes Citation7–10.
2-Amino-4H-pyran derivatives are important as they are often used in cosmetics and pigments, and as potentially biodegradable agrochemicals Citation11–13. Polyfunctionalized 4H-pyran structural motifs occur in a variety of natural products Citation14 Citation15 and also constitute biologically interesting compounds Citation11–13 Citation16–22. Pyranopyrazoles prevalently hold a position of prominence attributing to their biological activities which include insecticidal Citation23 anti-inflammatory Citation24, molluscicidal Citation25 Citation26, besides being identified as a screening kit for Chk1 kinase inhibitor Citation27 .
Different methods have been reported Citation27–41 for the synthesis of these derivatives. Recently, pyrano[2,3-c]pyrazoles have been synthesized using catalysts like triethyamine Citation28, Mg/Al hydrotalcite Citation29, glycine Citation30, and L-proline in [bmim]BF4 Citation31 whereas the synthesis of 2-amino-4H-pyrans has been reported using catalysts like Cu(II)oxymetasilicate Citation34, ZnO/MgO solid sample containing ZnO nanoparticles Citation35, silica nanoparticles Citation36, piperidine Citation37, magnesium oxide Citation38, or 1,1,3,3-tetramethylguanidine (TMG) Citation39 and also under microwaves exposure Citation40 in one pot reactions. Though there are many reports on the synthesis of 4H-pyrans and 4H-pyrano[2,3-c]pyrazoles. There is no report on the solvent-free synthesis of these derivatives using the same catalytic system.
Ionic liquids have emerged as a set of green solvents with unique properties such as tunable polarity, high thermal stability, and immiscibility with a number of organic solvents, negligible vapor pressure, and recyclability Citation42 Citation43. Task-specific ionic liquids further enhance the versatility of ionic liquids, by performing the dual role of solvent as well as catalysts in organic reactions Citation44–47. In continuation to our endeavor Citation48–50 aimed at developing green synthetic protocol, we decided to develop a general rapid, high yielding, environmentally benign protocol for the synthesis of 4H-pyrans and 4H-pyrano[2,3-c]pyrazoles using the task-specific basic ionic liquid (TSIL), [bmim]OH.
Results and discussion
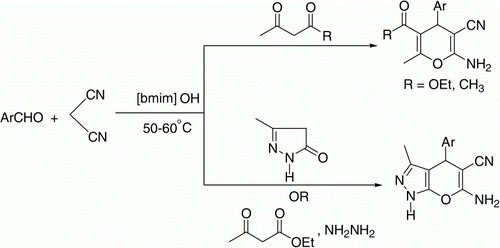
We herein present an efficient and facile protocol for the synthesis of 4H-pyrans from aldehydes, malononitrile, and ethyl acetoacetate/acetylacetone and also synthesis of 4H-pyrano[2,3-c]pyrazoles from three-component condensation of aldehydes, malononitrile, and pyrazolone or four-component condensation of aldehydes, malononitrile, ethyl acetoacetate, and hydrazine monohydrate using [bmim]OH as task-specific ionic liquid at 50–60°C.
The reaction of 4-chlorobenzaldehyde (1.0 mmol), malononitrile (1.0 mmol), and ethyl acetoacetate (1.0 mmol) was optimized by varying molar ratios of the TSIL [bmim]OH and temperature. It was observed that 20 mol% of [bmim]OH was most effective for the desired condensation reaction at 50–60°C yielding 91% of the 5-ethoxycarbonyl-2-amino-4-(4-chlorophenyl)-3-cyano-6-methyl-4H-pyran (1a) in 30 min without any catalyst (entries 1–4, ) unlike all previous reports. Higher amount of [bmim]OH did not effect the time or yield of the reaction (entry 5). However [bmim]Br and [bmim]BF4 were found to be ineffective for this condensation (entries 6–7, ). This confirms the vital role of the hydroxyl counter anion of [bmim]OH functionalized ionic liquid in this transformation. We, thus, chose to use 20 mol% of [bmim]OH to extend the protocol.
Table 1. Optimization of reaction conditions for the condensation of 4-chloro benzaldehyde, malononitrile, and ethyl acetoacetate.
Thereafter, a series of reactions were carried out using diversely substituted aldehydes under identical reaction conditions. All these aldehydes underwent three-component cyclo-condensation reaction with malononitrile and ethyl acetoacetate to produce 4H-pyran derivatives in excellent yields (entries 1–12, ) (Equation 1). All the functionalities were compatible under the reaction conditions. Reactions of aromatic aldehydes with electron-withdrawing groups such as chloro, nitro, proceeded at faster rates and gave better yields than those with electron donating groups such as methoxy and methyl.
Table 2. [bmim]OH catalyzed three-component synthesis of 5-acyl/alkoxycarbonyl-2-amino-4-aryl-3-cyano-6-methyl-4H-pyrans.
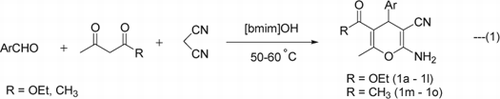
The replacement of ethyl acetoacetate by acetylacetone was also examined. In accord with our expectations, these reactions proceeded smoothly and afforded 5-acetyl-2-amino-4-aryl-3-cyano-6-methyl-4H-pyrans (entries 13–15, ) in good yield (Equation 1).
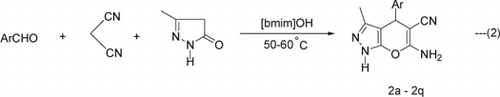
The scope of this protocol was then investigated for one pot cyclo-condensation of aldehydes, malononitrile, and 5-methyl-2,4-dihydropyrazol-3-one to afford 4H-pyrano[2,3-c]pyrazoles. To our delight, the reaction proceeded smoothly under above optimized conditions. A wide range of diversely substituted aromatic, and heteroaromatic aldehydes underwent this three-component cyclo-condensation with malononitrile and 5-methyl-2,4-dihydropyrazol-3-one to produce 4H-pyrano[2,3-c] pyrazoles (2a–2q) using 20 mol% of [bmim]OH as TSIL at 50–60°C (Equation 2). The reactions were completed in 5–10 min giving excellent yield of the products (Method A, ). The substituents in the aromatic ring did not show any significant effect on the rate of the reaction and yield of the products.
Table 3. Synthesis of 4H-pyrano[2,3-c]pyrazoles catalyzed by [bmim]OH via Methods A & B.
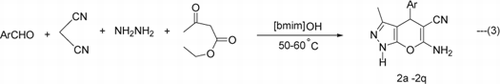
Furthermore, synthesis of 4H-pyrano[2,3-c]pyrazoles (2a–2q) could also be achieved via four-component cyclo-condensation of hydrazine monohydrate, ethyl acetoacetate, aldehydes, and malononitrile (Equation 3) using 20 mol% of TSIL [bmim]OH at 50–60°C (Method B). A comparison of the results (Method A and B) is drawn in . Though comparative yields were obtained by both methods, the reactions carried out via three-component condensation required shorter duration for completion. It should be noted that ethyl acetoacetate and hydrazine undergo condensation to give 5-methyl-2,4-dihydropyrazol-3-one.
Finally, the recycling of the basic ionic liquid [bmim]OH was investigated during the synthesis of 1a. After completion of the reaction, the solid product was collected by filtration. The filtrate containing the ionic liquid was rinsed with ether and further vacuumed to dryness at 90°C for 2 h to remove any trapped water, to afford [bmim]OH which was reused directly for the next run. It was found that the ionic liquid can be used for the reactions for up to three runs without any appreciable loss of efficiency.
Experimental
All the chemicals used were purchased from Sigma–Aldrich and were used as such. All products are known, and were identified by comparison of spectral and physical data with the literature. Melting points were determined on a melting point apparatus and are uncorrected. IR (KBr) spectra were recorded on Perkin–Elmer FTIR spectrophotometer. The 1H NMR spectra were recorded on Bruker Spectrospin spectrometer (300 MHz) and Jeol JNM ECX-400P (400 MHz) using TMS as an internal standard.
General procedure for the synthesis of 4H-pyran derivatives (1a–1o)
A mixture of aldehyde (1.0 mmol), malononitrile (1.0 mmol), ethyl acetoacetate or acetylacetone (1.0 mmol), and [bmim]OH (20 mol%) were stirred magnetically in an oil-bath maintained at 50–60°C for an appropriate time as mentioned in . After completion of the reaction as monitored by TLC using petroleum ether:ethyl acetate (60:40) as eluent, the reaction mixture was allowed to cool to room temperature and quenched with water (~5 mL). The solid product obtained was filtered, washed with water and dried. The crude product was washed well with hot ethanol to yield pure 4H-pyran derivatives.
General procedure for the synthesis of 4H-pyrano[2,3-c]pyrazoles (2a–2q)
Method A
A mixture of 5-methyl-2,4-dihydro-pyrazol-3-one (1 mmol), aldehyde (1 mmol), malononitrile (1 mmol), and [bmim]OH (20 mol%) was stirred and heated in an oil-bath maintained at 50–60°C for 5–10 min. Progress of the reaction was monitored by TLC using ethyl acetate:petroleum ether (60:40) as eluent. Upon completion of the reaction, water (~5 mL) was added and the contents were stirred. The solid product obtained was filtered, washed with water and dried to afford the crude product, which was recyrstallized from ethanol to yield pure 4H-pyrano[2,3-c]pyrazoles.
General procedure for the synthesis of 4H-pyrano[2,3-c]pyrazoles (2a–2q)
Method B
A mixture of hydrazine hydrate (96%) (1 mmol), ethyl acetoacetate (1 mmol), aldehyde (1 mmol), malononitrile (1 mmol), and [bmim]OH (20 mol%) was stirred at 50–60°C in an oil-bath for 10–15 min until completion of the reaction as monitored by TLC. The reaction was worked up as in Method A to yield pure products (2a–2q).
Representative analytical data
5-Ethoxycarbonyl-2-amino-4-(4-chlorophenyl)-3-cyano-6-methyl-4H-pyran (1a). White solid, 1H NMR(400 MHz, CDCl3): δH 7.27 (d, J=8.20 Hz, 2H, ArH), 7.15 (d, J=8.24 Hz, 2H, ArH), 4.53 (s, br, 2H, NH2), 4.42 (s, 1H, CH), 4.05 (q, J 1,3 =6.88 Hz, J 1,2 =3.44 Hz, 2H, OCH2), 2.37 (s, 3H,CH3), 1.13 (t, J=7.12 Hz, 3H, CH3); IR (KBr) (νmax, cm−1): 3410, 3333, 2193, 1693, 1595.
5-Acetyl-2-amino-3-cyano-6-methyl-4-(4-methoxyphenyl)-4H-pyran (1m). White solid, 1H NMR(400 MHz, CDCl3): δH 7.12 (d, J=8.8 Hz, 2H, ArH), 6.86 (d, J=8.8 Hz, 2H, ArH), 4.48 (s, br, 2H, NH2), 4.39 (s, 1H, CH), 3.78 (s, 3H, OCH3), 2.28 (s, 3H, CH3), 2.05 (s, 3H, CH3); IR (KBr) (νmax, cm−1): 3460, 3300, 3170.,2180, 1695,1660, 1600, 1510.
6-Amino-3-methyl-4-(4-nitrophenyl)-1,4-dihydro-pyrano[2,3-c]pyrazole-5-carbonitrile (2e). White solid, 1H NMR (300 MHz, DMSO-d6): δH 12.14 (s, 1H, NH), 8.19 (d, J=8.7 Hz, 2H, ArH), 7.45 (d, J=8.4 Hz, 2H, ArH), 6.96 (s, br, 2H, NH2), 4.80 (s, 1H, CH), 1.78 (s, 3H, CH3); IR (KBr) (νmax, cm−1): 3477, 3229, 3119, 2196, 1651, 1595, 1516, 1401.
Conclusion
In conclusion, we have demonstrated a novel, convenient, expeditious, and common protocol for the synthesis of 4H-pyrans and 4H-pyrano[2,3-c]pyrazoles in excellent yields, using the task-specific basic ionic liquid, [bmim]OH which can be recycled.
Acknowledgements
Ankita Chaudhary thanks C.S.I.R., New Delhi, India for the grant of Junior Research Fellowship.
References
- Ugi , I. Pure Appl. Chem . 2001 , 73 , 187 .
- Domling , A. Chem. Rev . 2006 , 106 , 17 – 89 .
- D'Souza , D.M. ; Mueller , T.J.J. Chem. Soc. Rev . 2007 , 36 , 3169 – 3210 .
- Cariou , C.C.A. ; Clarkson , G.J. ; Shipman , M. J. Org. Chem . 2008 , 73 , 9762 – 9764 .
- Alizadeh , A. ; Mobahedi , F. ; Esmaili , A. Tetrahedron Lett . 2006 , 47 , 4469 – 4471 .
- Umkeherer , M. ; Kalinski , C. ; Kolb , J. ; Burdack , C. Tetrahedron Lett . 2006 , 47 , 2391 – 2393 .
- Weber , L. Drug Discov. Today 2002 , 7 , 143 – 147 .
- Hulme , C. ; Gore , V. Curr. Med. Chem . 2003 , 10 , 51 – 80 .
- Tempest , P. A. Curr. Opin. Drug Discov. Dev . 2005 , 8 , 776 – 788 .
- Kalinski , C. ; Lemoine , H. ; Schmidt , J. ; Burdack , C. ; Kolb , J. ; Umkehrer , M. ; Ross , G. Synthesis 2008 , 4007 – 4011 .
- Morinaka , Y. ; Takahashi , K. Jpn Patent JP52017498 1977 ; Chem. Abstr . 1977 , 87 , 102299 .
- Witte , E. C. ; Neubert , P. ; Roesch , A. Ger Offen DE3427985, 1986 ; Chem. Abstr . 1986 , 104 , 224915f .
- Hafez , E. A. ; Elnagdi , M. H. ; Elagamey , A. A. ; El-Taweel , F. A. Heterocycles 1987 , 26 , 903 – 907 .
- Kuthan , J. Adv. Heterocycl. Chem . 1983 , 34 , 145 – 303 .
- Hatakeyama , S. ; Ochi , N. ; Numata , H. ; Takano , S. J. Chem. Soc. Chem. Commun . 1988 , 1202 – 1204 .
- Zamocka , J. ; Misikova , E. ; Durinda , J. Pharmazie 1991 , 46 , 610 – 613 .
- Wang , J. L. ; Liu , D. ; Zhang , Z. J. ; Shan , S. ; Han , X. ; Srinivasula , S. M. ; Croce , C. M. ; Alnemri , E. S. ; Huang , Z. Proc. Natl. Acad. Sci. U.S.A . 2007 , 97 , 7124 – 7129 .
- El-Saghier , A. M. M. ; Naili , M. B. ; Rammash , B. K. ; Saleh , N. A. ; Kreddan , K. M. ARKIVOC 2007 , 83 – 91 .
- Kumar , R. R. ; Perumal , S. ; Senthilkumar , P. ; Yogeeswari , P. ; Sriram , D. Bioorg. Med. Chem. Lett . 2007 , 17 , 6459 – 6462 .
- Fairlamb , I. J. S. ; Marrison , L. R. ; Dickinson , J. M. ; Lu , F.-J. ; Schmidt , J. P. Bioorg. Med. Chem . 2004 , 12 , 4285 – 4299 .
- Aytemir , M. D. ; Erol , D. D. ; Hider , R. C. ; Ozalp , M. Turk. J. Chem . 2003 , 27 , 757 – 764 .
- Kidwai , M. ; Saxena , S. ; Khan , M. K. R. ; Thukral , S. S. Bioorg. Med. Chem. Lett . 2005 , 15 , 4295 – 4298 .
- Tamany , E. S. ; El-Shahed , F. A. ; Mohamed , B. H. J. Serb. Chem. Soc . 1999 , 6 , 9 – 18 .
- Zaki , M. E. A. ; Soliman , H. A. ; Hiekal , O. A. ; Rashad , A. E. Z. Naturforsch. C . 2006 , 61 , 1 – 5 .
- Abdelrazek , F. M. ; Metz , P. ; Metwally , N. H. ; El-Mahrouky , S. F. Arch. Pharm . 2006 , 8 , 456 – 460 .
- Abdelrazek , F. M. ; Metz , P. ; Kataeva , O. ; Jaeger , A. ; El- Mahrouky , S. F. Arch. Pharm . 2007 , 340 , 543 – 548 .
- Foloppe , N. ; Fisher , L. M. ; Howes , R. ; Potter , A. ; Robertson , G. S. ; Surgenor , A. E. Bioorg. Med. Chem . 2006 , 14 , 4792 – 4802 .
- Litvinov , Y. M. ; Shestopalov , A. A. ; Rodinovskaya , L. A. ; Shestopalov , A. M. J. Comb. Chem . 2009 , 11 , 914 – 919 .
- Kshirsagar , S. W. ; Patil , N. R. ; Samant , S. D. Synth. Commun . 2011 , 41 , 1320 – 1325 .
- Reddy , M. B. M. ; Jayashankara , V. P. ; Pasha M. A. Synth. Commun . 2010 , 40 , 2930 – 2934 .
- Khurana , J. M. ; Nand , B. ; Kumar , S. Synth. Commun . 2011 , 41 , 405 – 410 .
- Peng , Y. ; Song , G. ; Dou , R. Green Chem . 2006 , 8 , 573 – 575 .
- Sharanina , L. G. ; Promonenkov , L. G. ; Puzanova , V. V. ; Sharanona , Y. A. Chem. Heterocycl. Compd . 1982 , 18 , 607 – 611 .
- Heravi , M. M. ; Beheshtiha , Y. S. ; Pirnia , Z. ; Sadjadi , S. ; Adibi , M. Synth. Commun . 2009 , 39 , 3663 – 3667 .
- Valizadeha , H. ; Azimib , A. A. J. Iran. Chem. Soc . 2011 , 8 , 123 – 130 .
- Banerjee , S. ; Horn , A. ; Khatri , H. ; Sereda , G. Tetrahedron Lett . 2011 , 52 , 1878 – 1881 .
- Lu , G.-P. ; Cai , C. J. Heterocycl. Chem . 2011 , 48 , 124 – 128 .
- Kumar , D. ; Reddy , V. B. ; Sharad , S. ; Dube , U. ; Kapur , S. ; Eur. J. Med. Chem . 2009 , 44 , 3805 – 3809 .
- Peng , Y. ; Song , G. ; Huang , F. Monatsh. Chem . 2005 , 136 , 727 – 731 .
- Peng , Y. ; Song , G. Catal. Commun . 2007 , 8 , 111 – 114 .
- Martin , N. ; Seoane , C. ; Soto , J. L. Tetrahedron 1988 , 44 , 5861 – 5868 .
- Welton , T. Chem. Rev . 1999 , 99 , 2071 – 2084 ; Welton, T. Coord. Chem. Rev. 2004, 248, 2459–2477 .
- Wasserscheid , P. ; Keim , W. ; Angew. Chem. Int. Ed . 2000 , 39 , 3772 – 3789 .
- Ranu , B. C. ; Banerjee , S. Org. Lett . 2005 , 7 , 3049 – 3052 .
- Xu , J. M. ; Liu , B. K. W. ; Wu , B. ; Qian , C. ; Wu , Q. ; Lin , X. F. J. Org. Chem . 2006 , 71 , 3991 – 3993 .
- Davoodnia , A. ; Heravi , M. M. ; Daghigh , L. R. ; Hoseini , N. T. Monatsh. Chem . 2009 , 140 , 1499 – 1502 .
- Heravi , M. M. ; Zakeri , M. ; Karimi , N. ; Saeedi , M. ; Oskooie , H. A. ; Hosieni ; N. T. Synth. Commun . 2010 , 40 , 1998 – 2006 .
- Khurana , J. M. ; Kumar , S. Tetrahedron. Lett . 2009 , 50 , 4125 – 4127 .
- Khurana , J. M. ; Magoo , D. Tetrahedron. Lett . 2009 , 50 , 4777 – 4780 ; Khurana, J. M.; Magoo, D. Tetrahedron. Lett. 2009, 50, 7300–7303 .
- Khurana , J. M. ; Nand , B. ; Saluja , P. Tetrahedron 2010 , 66 , 5637 – 5641 .