Abstract
Potassium permanganate (KMnO4) oxidizes quaternary ammonium bromides to the corresponding tribromides conveniently and effectively. The procedure adopted is environmentally friendly as it is solvent-free. High purity, excellent yield, shorter reaction time and mild reaction conditions are some of the advantages of this synthetic protocol. The efficacy of quaternary ammonium tribromides, especially benzyltrimethylammonium tribromide, is also studied for bromination of some organic substrates.
Introduction
Traditional methods of bromination, especially of aromatics involving use of elemental bromine, NBS, and so on suffer from several drawbacks such as use of hazardous and toxic solvents, difficulty in recovery of high boiling solvents, use of costly catalysts, use of chlorinated solvent, high reaction time Citation1–6. In recent past, organic quaternary ammonium tribromides (QATBs) have been reported to be highly efficient brominating as well as versatile oxidizing agents and allows a more selective bromination of organic compounds Citation7–9. The overwhelming numbers of reports Citation10–13 serve as a testimony to the unparalleled utility of QATBs in a variety of transformation of synthetic importance. As for example, these reagents are capable of brominating organic substrates without the use of elemental bromine which is associated with environmental hazards with respect to its transport, handling, and storage. They act as vital reagents for preparation of bromoorganics Citation8, and are capable of acting as flame retardants, agrochemicals and other specialty products Citation14, Citation15. They are widely used in the bromination of allyl alcohols, enones, alkenes, alkynes, activated aromatics and also as bifunctional catalyst in oxidative bromination of aromatic compounds. The main advantage of QATB is that they are crystalline, easy to handle and maintain the desired stoichiometry. Thus intrigued by the spectrum of uses of QATBs, several strategies for their syntheses have been introduced over the years. But most of the traditional methods of their preparations involve elemental bromine and in some cases hydrobromic acid which causes an environmental concern Citation10, Citation15, particularly in view of the recent environmental legislations on greener synthetic protocols Citation16–19. A few environmentally benign methods of syntheses of tribromides have been reported in last few years including some methods of our own group Citation7, Citation8, Citation10. Another recent method Citation13 worth mentioning involves the use of CAN as oxidant and water as solvent. These new methods are found to be attractive from environmental point of view as these methods minimize the negative effects encountered in the reported methodologies reported so far. Thus, although numerous methodologies have been developed there is still a need to find out better alternative.
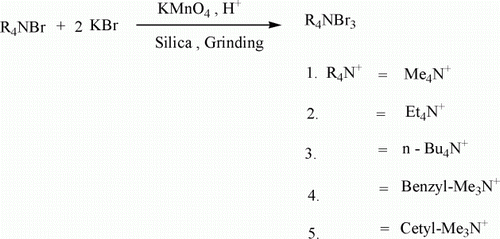
Therefore in continuation of our research interest to develop practical, simple and green methodologies in organic synthesis Citation20, we report herein a convenient route for the oxidative transformation of bromides to tribromides using KMnO4 as oxidizing agent. Potassium permanganate is known to be an inexpensive and environmentally friendly oxidant Citation21, Citation22. It has been used because of its proven effectiveness as an oxidant in organic chemistry for over a century. Oxidations carried out by KMnO4 have no adverse environmental impact because the recycling technology has made these processes environment-friendly and sustainable. Thus permanganate may be regarded as “A Green and Versatile Industrial Oxidant” Citation22. As far as our knowledge goes, KMnO4 has never been used as an oxidant for the synthesis of organic ammonium tribromides. Literature reports Citation23, Citation24 indicate that interaction of KMnO4 with organic ammonium bromide such as tetrabutyl ammonium bromide results in the formation of an organic solvent soluble oxidant tetrabutyl ammonium permanganate [(C4H9)4N+]. This reagent was conveniently used as organic soluble oxidant for oxidation of aldehydes to carboxylic acids, benzyl alcohol to benzoic acid, stilbene to benzoic acid. Our present method of synthesis of QATBs is one of the favorable methods as this reaction takes place in solid support silica without the use of any solvent and involves a very cheap and environmentally friendly oxidant ().
Results and discussion
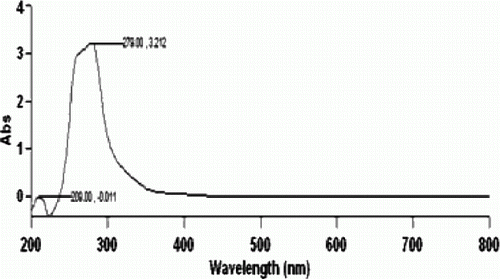
The synthesis of quaternary ammonium tribromides, QATBs involve grinding of a mixture of KMnO4, quaternary ammonium bromide, potassium bromide, and 4N H2SO4 supported on silica gel. Silica gel (60–120 mesh) was used to absorb the dilute sulphuric acid and did not form the silica-sulfuric acid catalyst. Sulfuric acid was found to be consumed at the end of the reaction. Potassium bromide in the reaction is used as a source of additional two equivalents of bromides. The grinding of the reaction mixture resulted in the immediate formation of yellow to orange-yellow coloration indicative of the formation of tribromide. The tribromide thus synthesized can be stored stable for a prolonged period. The tribromides were characterized by comparing their melting points with those of authentic samples. The UV–Visible spectrum of benzyltrimethylammonium tribromide, BTMATB in acetonitrile () was recorded which shows an intense absorption peak at 279 nm indicative of the presence of anion Citation25.
The oxidative conversion of bromide to tribromide by permanganate is believed to proceed with the formation of Br2 followed by combination of Br2 with Br−. The use of KBr and sulphuric acid generates HBr momentarily but the H+ ion is immediately utilized for oxidation of Br− to by Mn(VII) forming H2O in the process. Moreover, aqueous HBr is considered to be more toxic and environmentally unsafe than sulfuric acid which renders its direct use as an unsafe practice Citation7
,
Citation26.
Benzyltrimethylammonium tribromide (BTMATB) as brominating agent has not been studied extensively. This has prompted us to investigate the synthetic utility of BTMATB in bromination of some aromatic compounds. The experimental results of the bromination reactions of organic substrates with BTMATB are summarized in .
Table 1. Bromination of aromatic compounds with BTMATB.
All the bromination reactions were carried out at room temperature. The aromatics included polycyclic hydrocarbons like anthracene, sensitive substrate such as imidazole and activated aromatics such as aniline. BTMATB brominates aniline effectively in 50% aq. DMF to 4-bromo and 2,4,6-tribromoaniline selectively and in high yields depending on the molar ratio of brominating reagent. Similarly, anthracene can be easily brominated in acetic acid selectively to 9-bromo and 9,10-dibromo anthracene by maintaining the molar ratio between substrate and BTMATB at (1:1) and (1:2), respectively. Bromination was also carried out for hetero aromatics like imidazole in water–DMF mixture to afford 2,4,5-tribromoimidazole. BTMATB also brominates the double bond of cinnamic acid under mild condition to give 2,3-dibromo-3 phenyl cinnamic acid in moderate yield. Thus, synthetic protocol for bromination for aromatics and heteroaromatics by using BTMATB as brominating agent shows that its reactivity is comparable to other tribromides reported earlier Citation11, Citation27.
Experimental
All chemicals are commercially available and were used without further purification. The melting points of the synthesized compounds were determined in open capillaries and are uncorrected. The completion of the reaction was monitored by TLC. The products were further analyzed by FT-IR and 1H NMR and the spectral data were compared with those of authentic compounds Citation28. IR spectra were recorded on KBr with Perkin Elmer BX-FTIR spectrometer. 1H NMR spectra were recorded in DMSO-d6 using TMS as internal standard on a 400 MHz Varian spectrometer.
General procedure for preparation of quaternary ammonium tribromides (QATBs)
In a typical procedure BTMATB was prepared simply by mixing benzyl trimethyl ammonium bromide (1 mmol), potassium bromide (2 mmol), and KMnO4 (0.2 mmol) with 4 N sulphuric acid (0.75 ml) in a mortar. A little silica was added to the mixture to make it dry enough. This was then further mixed by grinding for ca. 5 minutes in the mortar. The orange-yellow product thus formed was extracted with 5 ml of ethyl acetate and concentrated under reduced pressure to get the tribromide in excellent yield with high purity. The isolated yield for BTMATB was 86% (mp: 99°C). The yields were approximately 75–85% for other quaternary ammonium tribromides, which include tetramethyl ammonium bromide 1 (82%), tetraethylammonium tribromide 2 (78%), tetrabutylammonium tribromide 3 (85%), and cetyltrimethylammonium tribromide 5 (83%).
General procedure for bromination of aromatic compounds with BTMATB
For monobrominated products, 1 mmol of the aromatic substrate was dissolved in 5 ml of the solvent (as mentioned in ). To this solution was added 1 mmol of BTMATB. The mixture was magnetically stirred at room temperature for the specified reaction period until the disappearance of the orange color of the solution and progress of the reaction was monitored by TLC. The brominated product was precipitated by adding water. The product was filtered in vacuo and washed with water. Similarly, for dibrominated and tribrominated products 2 mmol and 3 mmol of the BTMATB were taken, respectively. In case of anthracene, after the reaction was over, the products were extracted with diethyl ether. The organic layer was separated, washed with water and aqueous NaHCO3 solution and finally dried over anhydrous sodium sulfate. The solvent was then evaporated to obtain the desired products. For imidazole, the substrate was treated with BTMATB along with 2 mmol of CaCO3 to afford 2,4,5-tribromoimidazole in 40% yield.
Spectral data of selected compounds
4-Bromoaniline (, entry 1): IR (KBr) 3375, 3032, 2925, 1629, 1491, 1281, 1178, 1071, 810, 605, 502 cm−1. 1H NMR (400 MHz, DMSO-d6, δppm): 3.65 (brs, 2H), 6.55 (d, J=8.4 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H).
9-Bromoanthracene (, entry 4): IR (KBr) 3049, 2964, 2861, 1678, 1525, 1378, 1260, 1151, 952, 884, 838, 808 cm−1. 1H NMR (400 MHz, DMSO-d6, δppm): 7.62 (m, 2H), 7.71 (m, 2H), 8.15 (d, 2H), 8.41 (d, 2H), 8.70 (s, 1H).
9,10-Dibromoanthracene (, entry 5):IR (KBr) 1622, 1438, 1268, 927, 747, 578 cm−1.
1H NMR (400 MHz, DMSO-d6, δppm): 8.55(d, 4H), 7.59(d, 4H).
4-Bromoacetanilide (, entry 6): IR (KBr) 3293, 3257, 3191, 3058, 1670, 1603, 1537, 1491, 1393, 1312, 1020, 820, 743 cm−1. 1H NMR (400 MHz, DMSO-d6, δppm): 2.02 (s, 2H), 7.44 (d, J=8.4 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 10.05 (s, 1H).
Conclusion
The method presented here serves as a green alternative to some of the already known protocols for the syntheses of quaternary ammonium tribromides. The method is very facile, cost effective and ecofriendly. It provides products in high yield with no waste being generated.
Acknowledgements
S.S.D. acknowledges the Department of Science and Technology, DST, New Delhi, India, for financial assistance received through a SERC fast track project (SR/FTP/CS-100/2007).
References
- Kumar , L. ; Sharma , V. ; Mahajan , T. ; Agarwal , D.D. Org. Proc. Res. Dev . 2010 , 14 , 174 – 179 .
- Chiappe , C. ; Leandri , E. ; Pieraccini , D. Chem. Commun . 2004 , 22 , 2536 – 2537 .
- Tajik , H. ; Shirini , F. ; Zadeh , P.H. ; Rashtabadi , H.R. Synth. Commun . 2005 , 35 , 1947 – 1952 .
- Rahman , H. ; Mahood , T. ; Maryam , M. ; Zahra , L. Synth. Commun . 2010 , 40 , 868 – 876 .
- Raju , T. ; Kalangiappar , K. ; Kulandainathan , M.A. ; Uma , U. ; Malini , R. ; Muthukumaran , A. Tetrahedron Lett . 2006 , 47 , 4581 – 4584 .
- Sels , B.F. ; Devos , E.D. ; Jacobs , P.A. J. Am. Chem. Soc . 2001 , 123 , 8350 – 8359 .
- Chaudhuri , M.K. ; Bora , U. ; Dehury , S.K. ; Dey , D. ; Dhar , S.S. ; Kharmawphlang , W. ; Choudhary , B.M. ; Mannepalli , L.K. US Patent 7,005,548 , February 28 2006 .
- Bora , U. ; Chaudhuri , M.K. ; Dey , D. ; Dhar , S.S. Pure Appl. Chem . 2001 , 73 , 93 – 102 .
- Pravst , I. ; Zupan , M. ; Stavber , S. Curr. Org. Chem . 2009 , 13 , 47 – 70 .
- Bora , U. ; Bose , G. ; Chaudhuri , M.K. ; Dhar , S.S. ; Gopinath , R. ; Khan , A.T. ; Patel , B.K. Org. Lett . 2000 , 2 , 247 – 249 .
- Chaudhuri , M.K. ; Khan , A.T. ; Patel , B.K. Tetrahedron Lett . 1998 , 39 , 8163 – 8166 .
- Amosava , S.V. ; Abramova , E.V. ; Potapov , V.A. Russian J. Chem . 2008 , 78 , 146 .
- Borah , R. ; Thakur , A.J. Synth. Commun . 2007 , 37 , 933 – 939 .
- Adimurthy , S. ; Ghosh , S. ; Patoliya , P.U. ; Ramachandraiah , G. ; Agrawal , M. ; Gandhi , M.R. ; Upadhyay , S.C. ; Ghosh , P.K. ; Ranu , B.C. ; Green Chem . 2008 , 10 , 232 – 237 .
- Wu , L.Q. ; Yang , C.C. J. Chin. Chem. Soc . 2009 , 56 , 606 – 608 .
- Anastas , P.T. ; Warner , J.C. Green Chemistry: Theory and Practice ; Oxford University : Oxford , , UK , 2000 ; pp 20 – 33 .
- Metzger , J.O. Angew. Chem. Int. Ed . 1998 , 37 , 2975 – 2978 .
- Clark , J.K. Green Chem . 1999 , 1 , 1 – 8 .
- Tanaka . K. ; Toda . F. Chem. Rev . 2000 , 100 , 1025 – 1074 .
- Dey , M. , Deb , K. , Dhar , S.S. Chin. Chem. Lett . 2011 , 22 , 296 – 299 .
- Shaabani , A. ; Lee , D.G. Tetrahedron Lett . 2001 , 42 , 5833 – 5836 .
- Singh , N. ; Lee , D.G. Org. Proc. Res. Dev . 2001 , 5 , 599 – 603 .
- Sala , T. ; Sargent , M.V. J. Chem. Soc., Chem. Comm . 1978 , 6 , 253 – 254 .
- Mishra , B.K. ; Sahu , S. ; Pradhan , S. ; Patel , S. Ind. J. Chem . 2009 , 48A , 1527 – 1531 .
- Thompson , R.C. Inorg. Bioinorg. Mech . 1986 , 4 , 65 .
- Kar , G. ; Saikia , A.K. ; Bora , U. ; Dehury , S.K. ; Chaudhuri , M.K. ; Tetrahedron Lett . 2003 , 44 , 4503 – 4505 .
- Hajipora , A.R. ; Imanieh , H. ; Pourmousavi , S.A. Synth. Commun . 2004 , 34 , 4597 – 4604 .
- Buckingham , J. , McDonald , F. .; Dictionary of Organic Compounds , 6th ed , Chapman & Hall : London , 1996 .